Iron Metabolism in Pancreatic Beta-Cell Function and Dysfunction
- PMID: 34831062
- PMCID: PMC8616520
- DOI: 10.3390/cells10112841
Iron Metabolism in Pancreatic Beta-Cell Function and Dysfunction
Abstract
Iron is an essential element involved in a variety of physiological functions. In the pancreatic beta-cells, being part of Fe-S cluster proteins, it is necessary for the correct insulin synthesis and processing. In the mitochondria, as a component of the respiratory chain, it allows the production of ATP and reactive oxygen species (ROS) that trigger beta-cell depolarization and potentiate the calcium-dependent insulin release. Iron cellular content must be finely tuned to ensure the normal supply but also to prevent overloading. Indeed, due to the high reactivity with oxygen and the formation of free radicals, iron excess may cause oxidative damage of cells that are extremely vulnerable to this condition because the normal elevated ROS production and the paucity in antioxidant enzyme activities. The aim of the present review is to provide insights into the mechanisms responsible for iron homeostasis in beta-cells, describing how alteration of these processes has been related to beta-cell damage and failure. Defects in iron-storing or -chaperoning proteins have been detected in diabetic conditions; therefore, the control of iron metabolism in these cells deserves further investigation as a promising target for the development of new disease treatments.
Keywords: Iron metabolism; beta-cell function; diabetes; reactive oxygen species.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
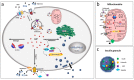
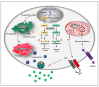
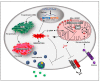
References
-
- Herrmann T., Muckenthaler M., van der Hoeven F., Brennan K., Gehrke S.G., Hubert N., Sergi C., Gröne H.-J., Kaiser I., Gosch I., et al. Iron Overload in Adult Hfe-Deficient Mice Independent of Changes in the Steady-State Expression of the Duodenal Iron Transporters DMT1 and Ireg1/Ferroportin. J. Mol. Med. 2004;82:39–48. doi: 10.1007/s00109-003-0508-x. - DOI - PubMed
-
- Fergelot P., Orhant M., Thénié A., Loyer P., Ropert-Bouchet M., Lohyer S., Le Gall J.-Y., Mosser J. Over-Expression of Wild-Type and Mutant HFE in a Human Melanocytic Cell Line Reveals an Intracellular Bridge between MHC Class I Pathway and Transferrin Iron Uptake. Biol. Cell. 2003;95:243–255. doi: 10.1016/S0248-4900(03)00057-1. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

