Fibroblast Memory in Development, Homeostasis and Disease
- PMID: 34831065
- PMCID: PMC8616330
- DOI: 10.3390/cells10112840
Fibroblast Memory in Development, Homeostasis and Disease
Abstract
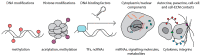
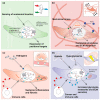
Fibroblasts are the major cell population in the connective tissue of most organs, where they are essential for their structural integrity. They are best known for their role in remodelling the extracellular matrix, however more recently they have been recognised as a functionally highly diverse cell population that constantly responds and adapts to their environment. Biological memory is the process of a sustained altered cellular state and functions in response to a transient or persistent environmental stimulus. While it is well established that fibroblasts retain a memory of their anatomical location, how other environmental stimuli influence fibroblast behaviour and function is less clear. The ability of fibroblasts to respond and memorise different environmental stimuli is essential for tissue development and homeostasis and may become dysregulated in chronic disease conditions such as fibrosis and cancer. Here we summarise the four emerging key areas of fibroblast adaptation: positional, mechanical, inflammatory, and metabolic memory and highlight the underlying mechanisms and their implications in tissue homeostasis and disease.
Keywords: biological memory; cancer; cell fate; epigenetic modification; fibroblasts; fibrosis; inflammation; mechanical stress; metabolism; positional identity; wound healing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Muhl L., Genové G., Leptidis S., Liu J., He L., Mocci G., Sun Y., Gustafsson S., Buyandelger B., Chivukula I.V., et al. Single-Cell Analysis Uncovers Fibroblast Heterogeneity and Criteria for Fibroblast and Mural Cell Identification and Discrimination. Nat. Commun. 2020;11:3953. doi: 10.1038/s41467-020-17740-1. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

