Milk Exosome-Derived MicroRNA-2478 Suppresses Melanogenesis through the Akt-GSK3β Pathway
- PMID: 34831071
- PMCID: PMC8616206
- DOI: 10.3390/cells10112848
Milk Exosome-Derived MicroRNA-2478 Suppresses Melanogenesis through the Akt-GSK3β Pathway
Abstract
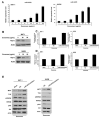
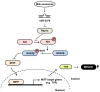
Exosomes participate in intercellular communication by transferring molecules from donor to recipient cells. Exosomes are found in various body fluids, including blood, urine, cerebrospinal fluid and milk. Milk exosomes contain many endogenous microRNA molecules. MicroRNAs are small noncoding RNAs and have important roles in biological processes. The specific biological functions of milk exosomes are not well understood. In this study, we investigated the effects of milk exosomes on melanin production in melanoma cells and melanocytes. We found that milk exosomes decreased melanin contents, tyrosinase activity and the expression of melanogenesis-related genes in melanoma cells and melanocytes. Bovine-specific miR-2478 in exosomes inhibited melanin production. We found that Rap1a is a direct target gene of miR-2478 in melanoma cells and melanocytes. MiR-2478 overexpression decreased Rap1a expression, which led to downregulated melanin production and expression of melanogenesis-related genes. Inhibition of Rap1a expression decreased melanogenesis through the Akt-GSK3β signal pathway. These results support the role of miR-2478 derived from milk exosomes as a regulator of melanogenesis through direct targeting of Rap1a. These results show that milk exosomes could be useful cosmeceutical ingredients to improve whitening.
Keywords: Rap1a; melanogenesis; miR-2478; milk exosome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

