Insights into COVID-19 Vaccine Development Based on Immunogenic Structural Proteins of SARS-CoV-2, Host Immune Responses, and Herd Immunity
- PMID: 34831172
- PMCID: PMC8616290
- DOI: 10.3390/cells10112949
Insights into COVID-19 Vaccine Development Based on Immunogenic Structural Proteins of SARS-CoV-2, Host Immune Responses, and Herd Immunity
Abstract
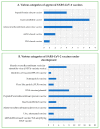
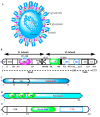
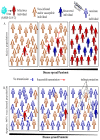
The first quarter of the 21st century has remarkably been characterized by a multitude of challenges confronting human society as a whole in terms of several outbreaks of infectious viral diseases, such as the 2003 severe acute respiratory syndrome (SARS), China; the 2009 influenza H1N1, Mexico; the 2012 Middle East respiratory syndrome (MERS), Saudi Arabia; and the ongoing coronavirus disease 19 (COVID-19), China. COVID-19, caused by SARS-CoV-2, reportedly broke out in December 2019, Wuhan, the capital of China's Hubei province, and continues unabated, leading to considerable devastation and death worldwide. The most common target organ of SARS-CoV-2 is the lungs, especially the bronchial and alveolar epithelial cells, culminating in acute respiratory distress syndrome (ARDS) in severe patients. Nevertheless, other tissues and organs are also known to be critically affected following infection, thereby complicating the overall aetiology and prognosis. Excluding H1N1, the SARS-CoV (also referred as SARS-CoV-1), MERS, and SARS-CoV-2 are collectively referred to as coronaviruses, and taxonomically placed under the realm Riboviria, order Nidovirales, suborder Cornidovirineae, family Coronaviridae, subfamily Orthocoronavirinae, genus Betacoronavirus, and subgenus Sarbecovirus. As of 23 September 2021, the ongoing SARS-CoV-2 pandemic has globally resulted in around 229 million and 4.7 million reported infections and deaths, respectively, apart from causing huge psychosomatic debilitation, academic loss, and deep economic recession. Such an unprecedented pandemic has compelled researchers, especially epidemiologists and immunologists, to search for SARS-CoV-2-associated potential immunogenic molecules to develop a vaccine as an immediate prophylactic measure. Amongst multiple structural and non-structural proteins, the homotrimeric spike (S) glycoprotein has been empirically found as the most suitable candidate for vaccine development owing to its immense immunogenic potential, which makes it capable of eliciting both humoral and cell-mediated immune responses. As a consequence, it has become possible to design appropriate, safe, and effective vaccines, apart from related therapeutic agents, to reduce both morbidity and mortality. As of 23 September 2021, four vaccines, namely, Comirnaty, COVID-19 vaccine Janssen, Spikevax, and Vaxzevria, have received the European Medicines Agency's (EMA) approval, and around thirty are under the phase three clinical trial with emergency authorization by the vaccine-developing country-specific National Regulatory Authority (NRA). In addition, 100-150 vaccines are under various phases of pre-clinical and clinical trials. The mainstay of global vaccination is to introduce herd immunity, which would protect the majority of the population, including immunocompromised individuals, from infection and disease. Here, we primarily discuss category-wise vaccine development, their respective advantages and disadvantages, associated efficiency and potential safety aspects, antigenicity of SARS-CoV-2 structural proteins and immune responses to them along with the emergence of SARS-CoV-2 VOC, and the urgent need of achieving herd immunity to contain the pandemic.
Keywords: SARS-CoV-2; coronavirus disease 19; herd immunity; immune response; infectious disease; pandemic; vaccination.
Conflict of interest statement
Authors declare no conflict of interest for this article.
Figures

References
-
- Bosco-Lauth A.M., Hartwig A.E., Porter S.M., Gordy P.W., Nehring M., Byas A.D., VandeWoude S., Ragan I.K., Maison R.M., Bowen R.A. Experimental infection of domestic dogs and cats with SARS-CoV-2: Pathogenesis, transmission, and response to reexposure in cats. Proc. Natl. Acad. Sci. USA. 2020;117:26382–26388. doi: 10.1073/pnas.2013102117. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

