Recent Advances in Understanding the Pathogenesis of Rheumatoid Arthritis: New Treatment Strategies
- PMID: 34831240
- PMCID: PMC8616543
- DOI: 10.3390/cells10113017
Recent Advances in Understanding the Pathogenesis of Rheumatoid Arthritis: New Treatment Strategies
Abstract
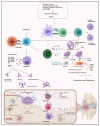
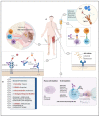
Rheumatoid arthritis (RA) is considered a chronic systemic, multi-factorial, inflammatory, and progressive autoimmune disease affecting many people worldwide. While patients show very individual courses of disease, with RA focusing on the musculoskeletal system, joints are often severely affected, leading to local inflammation, cartilage destruction, and bone erosion. To prevent joint damage and physical disability as one of many symptoms of RA, early diagnosis is critical. Auto-antibodies play a pivotal clinical role in patients with systemic RA. As biomarkers, they could help to make a more efficient diagnosis, prognosis, and treatment decision. Besides auto-antibodies, several other factors are involved in the progression of RA, such as epigenetic alterations, post-translational modifications, glycosylation, autophagy, and T-cells. Understanding the interplay between these factors would contribute to a deeper insight into the causes, mechanisms, progression, and treatment of the disease. In this review, the latest RA research findings are discussed to better understand the pathogenesis, and finally, treatment strategies for RA therapy are presented, including both conventional approaches and new methods that have been developed in recent years or are currently under investigation.
Keywords: auto-antibodies; autophagy; biological agents; citrullination; epigenetic; inflammation; phytochemical; rheumatoid arthritis; treatment.
Conflict of interest statement
The authors declare no conflict of interest. Figures were created with BioRender software (
Figures
References
-
- Sieghart D., Platzer A., Studenic P., Alasti F., Grundhuber M., Swiniarski S., Horn T., Haslacher H., Blüml S., Smolen J. Determination of autoantibody isotypes increases the sensitivity of serodiagnostics in rheumatoid arthritis. Front. Immunol. 2018;9:876. doi: 10.3389/fimmu.2018.00876. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

