Disruption of Mitochondrial Homeostasis: The Role of PINK1 in Parkinson's Disease
- PMID: 34831247
- PMCID: PMC8616241
- DOI: 10.3390/cells10113022
Disruption of Mitochondrial Homeostasis: The Role of PINK1 in Parkinson's Disease
Abstract
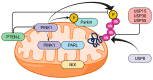
The progressive reduction of the dopaminergic neurons of the substantia nigra is the fundamental process underlying Parkinson's disease (PD), while the mechanism of susceptibility of this specific neuronal population is largely unclear. Disturbances in mitochondrial function have been recognized as one of the main pathways in sporadic PD since the finding of respiratory chain impairment in animal models of PD. Studies on genetic forms of PD have provided new insight on the role of mitochondrial bioenergetics, homeostasis, and autophagy. PINK1 (PTEN-induced putative kinase 1) gene mutations, although rare, are the second most common cause of recessively inherited early-onset PD, after Parkin gene mutations. Our knowledge of PINK1 and Parkin function has increased dramatically in the last years, with the discovery that a process called mitophagy, which plays a key role in the maintenance of mitochondrial health, is mediated by the PINK1/Parkin pathway. In vitro and in vivo models have been developed, supporting the role of PINK1 in synaptic transmission, particularly affecting dopaminergic neurons. It is of paramount importance to further define the role of PINK1 in mitophagy and mitochondrial homeostasis in PD pathogenesis in order to delineate novel therapeutic targets.
Keywords: PINK1; Parkin; Parkinson’s disease; mitochondrial quality control; mitophagy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

