Vascular Dysfunction in Preeclampsia
- PMID: 34831277
- PMCID: PMC8616535
- DOI: 10.3390/cells10113055
Vascular Dysfunction in Preeclampsia
Abstract
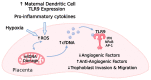
Preeclampsia is a life-threatening pregnancy-associated cardiovascular disorder characterized by hypertension and proteinuria at 20 weeks of gestation. Though its exact underlying cause is not precisely defined and likely heterogenous, a plethora of research indicates that in some women with preeclampsia, both maternal and placental vascular dysfunction plays a role in the pathogenesis and can persist into the postpartum period. Potential abnormalities include impaired placentation, incomplete spiral artery remodeling, and endothelial damage, which are further propagated by immune factors, mitochondrial stress, and an imbalance of pro- and antiangiogenic substances. While the field has progressed, current gaps in knowledge include detailed initial molecular mechanisms and effective treatment options. Newfound evidence indicates that vasopressin is an early mediator and biomarker of the disorder, and promising future therapeutic avenues include mitigating mitochondrial dysfunction, excess oxidative stress, and the resulting inflammatory state. In this review, we provide a detailed overview of vascular defects present during preeclampsia and connect well-established notions to newer discoveries at the molecular, cellular, and whole-organism levels.
Keywords: blood pressure; gestation; hypertension; placenta; preeclampsia; pregnancy; trophoblast; vessel.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

