Apparent Yield Stress of Sputum as a Relevant Biomarker in Cystic Fibrosis
- PMID: 34831330
- PMCID: PMC8619720
- DOI: 10.3390/cells10113107
Apparent Yield Stress of Sputum as a Relevant Biomarker in Cystic Fibrosis
Abstract
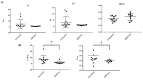
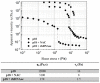
The mucus obstructing the airways of Cystic Fibrosis (CF) patients is a yield stress fluid. Linear and non-linear rheological analyses of CF sputa can provide relevant biophysical markers, which could be used for the management of this disease. Sputa were collected from CF patients either without any induction or following an aerosol treatment with the recombinant human DNAse (rhDNAse, Pulmozyme®). Several sample preparations were considered and multiple measurements were performed in order to assess both the repeatability and the robustness of the rheological measurements. The linear and non-linear rheological properties of all CF sputa were characterized. While no correlation between oscillatory shear linear viscoelastic properties and clinical data was observed, the steady shear flow data showed that the apparent yield stress of sputum from CF patients previously treated with rhDNAse was approximately one decade lower than that of non-treated CF patients. Similar results were obtained with sputa from non-induced CF patients subjected ex vivo to a Pulmozyme® aerosol treatment. The results demonstrate that the apparent yield stress of patient sputa is a relevant predictive/prognostic biomarker in CF patients and could help in the development of new mucolytic agents.
Keywords: Cystic Fibrosis; airway mucus; biomarker; rheology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Impact of PEGylation on the mucolytic activity of recombinant human deoxyribonuclease I in cystic fibrosis sputum.Clin Sci (Lond). 2018 Jul 18;132(13):1439-1452. doi: 10.1042/CS20180315. Print 2018 Jul 18. Clin Sci (Lond). 2018. PMID: 29871879
-
Effects of sputum oscillations and rhDNase in vitro: a combined approach to treat cystic fibrosis lung disease.Pediatr Pulmonol. 1998 Oct;26(4):250-5. doi: 10.1002/(sici)1099-0496(199810)26:4<250::aid-ppul3>3.0.co;2-x. Pediatr Pulmonol. 1998. PMID: 9811074
-
In vivo effects of recombinant human DNase I on sputum in patients with cystic fibrosis.Thorax. 1996 Feb;51(2):119-25. doi: 10.1136/thx.51.2.119. Thorax. 1996. PMID: 8711640 Free PMC article. Clinical Trial.
-
Mucus, phlegm, and sputum in cystic fibrosis.Respir Care. 2009 Jun;54(6):726-32; discussion 732. doi: 10.4187/002013209790983269. Respir Care. 2009. PMID: 19467160 Review.
-
Mucus structure and properties in cystic fibrosis.Paediatr Respir Rev. 2007 Mar;8(1):4-7. doi: 10.1016/j.prrv.2007.02.004. Epub 2007 Mar 21. Paediatr Respir Rev. 2007. PMID: 17419972 Review.
Cited by
-
Modeling Cystic Fibrosis Chronic Infection Using Engineered Mucus-like Hydrogels.ACS Biomater Sci Eng. 2024 Oct 14;10(10):6558-6568. doi: 10.1021/acsbiomaterials.4c01271. Epub 2024 Sep 19. ACS Biomater Sci Eng. 2024. PMID: 39297972
-
Mucus Structure, Viscoelastic Properties, and Composition in Chronic Respiratory Diseases.Int J Mol Sci. 2024 Feb 5;25(3):1933. doi: 10.3390/ijms25031933. Int J Mol Sci. 2024. PMID: 38339210 Free PMC article. Review.
-
Interplay between environmental yielding and dynamic forcing modulates bacterial growth.Biophys J. 2024 Apr 16;123(8):957-967. doi: 10.1016/j.bpj.2024.03.008. Epub 2024 Mar 7. Biophys J. 2024. PMID: 38454600 Free PMC article.
-
Aerosol-Mediated Non-Viral Lung Gene Therapy: The Potential of Aminoglycoside-Based Cationic Liposomes.Pharmaceutics. 2021 Dec 23;14(1):25. doi: 10.3390/pharmaceutics14010025. Pharmaceutics. 2021. PMID: 35056921 Free PMC article.
-
Rheological comparison of sputum and reconstituted airway epithelium mucus.Sci Rep. 2024 Dec 30;14(1):31660. doi: 10.1038/s41598-024-80932-y. Sci Rep. 2024. PMID: 39738355 Free PMC article.
References
-
- Middleton P.G., Mall M.A., Dřevínek P., Lands L.C., McKone E.F., Polineni D., Ramsey B.W., Taylor-Cousar J.L., Tullis E., Vermeulen F., et al. Elexacaftor–Tezacaftor–Ivacaftor for Cystic Fibrosis with a Single Phe508del Allele. N. Engl. J. Med. 2019;381:1809–1819. doi: 10.1056/NEJMoa1908639. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

