L-Arginine Ameliorates Defective Autophagy in GM2 Gangliosidoses by mTOR Modulation
- PMID: 34831346
- PMCID: PMC8619250
- DOI: 10.3390/cells10113122
L-Arginine Ameliorates Defective Autophagy in GM2 Gangliosidoses by mTOR Modulation
Abstract
Aims: Tay-Sachs and Sandhoff diseases (GM2 gangliosidosis) are autosomal recessive disorders of lysosomal function that cause progressive neurodegeneration in infants and young children. Impaired hydrolysis catalysed by β-hexosaminidase A (HexA) leads to the accumulation of GM2 ganglioside in neuronal lysosomes. Despite the storage phenotype, the role of autophagy and its regulation by mTOR has yet to be explored in the neuropathogenesis. Accordingly, we investigated the effects on autophagy and lysosomal integrity using skin fibroblasts obtained from patients with Tay-Sachs and Sandhoff diseases.
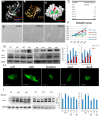
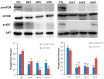
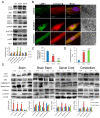
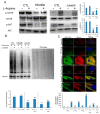
Results: Pathological autophagosomes with impaired autophagic flux, an abnormality confirmed by electron microscopy and biochemical studies revealing the accelerated release of mature cathepsins and HexA into the cytosol, indicating increased lysosomal permeability. GM2 fibroblasts showed diminished mTOR signalling with reduced basal mTOR activity. Accordingly, provision of a positive nutrient signal by L-arginine supplementation partially restored mTOR activity and ameliorated the cytopathological abnormalities.
Innovation: Our data provide a novel molecular mechanism underlying GM2 gangliosidosis. Impaired autophagy caused by insufficient lysosomal function might represent a new therapeutic target for these diseases.
Conclusions: We contend that the expression of autophagy/lysosome/mTOR-associated molecules may prove useful peripheral biomarkers for facile monitoring of treatment of GM2 gangliosidosis and neurodegenerative disorders that affect the lysosomal function and disrupt autophagy.
Keywords: GM2 gangliosidosis; L-arginine; autophagy; mTOR.
Conflict of interest statement
All the authors declare that no conflict of interest exists for any of them.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

