Reciprocal Regulation of Hippo and WBP2 Signalling-Implications in Cancer Therapy
- PMID: 34831354
- PMCID: PMC8625973
- DOI: 10.3390/cells10113130
Reciprocal Regulation of Hippo and WBP2 Signalling-Implications in Cancer Therapy
Abstract
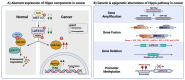
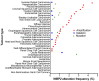
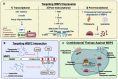
Cancer is a global health problem. The delineation of molecular mechanisms pertinent to cancer initiation and development has spurred cancer therapy in the form of precision medicine. The Hippo signalling pathway is a tumour suppressor pathway implicated in a multitude of cancers. Elucidation of the Hippo pathway has revealed an increasing number of regulators that are implicated, some being potential therapeutic targets for cancer interventions. WW domain-binding protein 2 (WBP2) is an oncogenic transcriptional co-factor that interacts, amongst others, with two other transcriptional co-activators, YAP and TAZ, in the Hippo pathway. WBP2 was recently discovered to modulate the upstream Hippo signalling components by associating with LATS2 and WWC3. Exacerbating the complexity of the WBP2/Hippo network, WBP2 itself is reciprocally regulated by Hippo-mediated microRNA biogenesis, contributing to a positive feedback loop that further drives carcinogenesis. Here, we summarise the biological mechanisms of WBP2/Hippo reciprocal regulation and propose therapeutic strategies to overcome Hippo defects in cancers through targeting WBP2.
Keywords: WW-domain binding protein 2; cancer; hippo pathway; oncogene; reciprocal regulation; therapeutics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

