Mitochondrial Dysfunction in Cancer Cachexia: Impact on Muscle Health and Regeneration
- PMID: 34831373
- PMCID: PMC8621344
- DOI: 10.3390/cells10113150
Mitochondrial Dysfunction in Cancer Cachexia: Impact on Muscle Health and Regeneration
Abstract
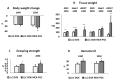
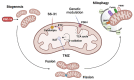
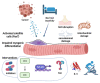
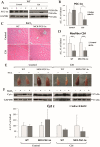
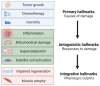
Cancer cachexia is a frequently neglected debilitating syndrome that, beyond representing a primary cause of death and cancer therapy failure, negatively impacts on patients' quality of life. Given the complexity of its multisystemic pathogenesis, affecting several organs beyond the skeletal muscle, defining an effective therapeutic approach has failed so far. Revamped attention of the scientific community working on cancer cachexia has focused on mitochondrial alterations occurring in the skeletal muscle as potential triggers of the complex metabolic derangements, eventually leading to hypercatabolism and tissue wasting. Mitochondrial dysfunction may be simplistically viewed as a cause of energy failure, thus inducing protein catabolism as a compensatory mechanism; however, other peculiar cachexia features may depend on mitochondria. On the one side, chemotherapy also impacts on muscle mitochondrial function while, on the other side, muscle-impaired regeneration may result from insufficient energy production from damaged mitochondria. Boosting mitochondrial function could thus improve the energetic status and chemotherapy tolerance, and relieve the myogenic process in cancer cachexia. In the present work, a focused review of the available literature on mitochondrial dysfunction in cancer cachexia is presented along with preliminary data dissecting the potential role of stimulating mitochondrial biogenesis via PGC-1α overexpression in distinct aspects of cancer-induced muscle wasting.
Keywords: PGC-1α; cancer cachexia; metabolism; mitochondria; muscle wasting; myogenesis; regeneration.
Conflict of interest statement
The authors have no relevant conflict of interest to disclose.
Figures
References
-
- Muscaritoli M., Lucia S., Farcomeni A., Lorusso V., Saracino V., Barone C., Plastino F., Gori S., Magarotto R., Carteni G., et al. Prevalence of malnutrition in patients at first medical oncology visit: The PreMiO study. Oncotarget. 2017;8:79884–79896. doi: 10.18632/oncotarget.20168. - DOI - PMC - PubMed

