Macrophage Reprogramming and Cancer Therapeutics: Role of iNOS-Derived NO
- PMID: 34831416
- PMCID: PMC8624911
- DOI: 10.3390/cells10113194
Macrophage Reprogramming and Cancer Therapeutics: Role of iNOS-Derived NO
Abstract
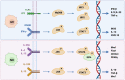
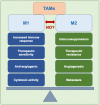
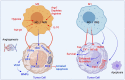
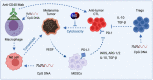
Nitric oxide and its production by iNOS is an established mechanism critical to tumor promotion or suppression. Macrophages have important roles in immunity, development, and progression of cancer and have a controversial role in pro- and antitumoral effects. The tumor microenvironment consists of tumor-associated macrophages (TAM), among other cell types that influence the fate of the growing tumor. Depending on the microenvironment and various cues, macrophages polarize into a continuum represented by the M1-like pro-inflammatory phenotype or the anti-inflammatory M2-like phenotype; these two are predominant, while there are subsets and intermediates. Manipulating their plasticity through programming or reprogramming of M2-like to M1-like phenotypes presents the opportunity to maximize tumoricidal defenses. The dual role of iNOS-derived NO also influences TAM activity by repolarization to tumoricidal M1-type phenotype. Regulatory pathways and immunomodulation achieve this through miRNA that may inhibit the immunosuppressive tumor microenvironment. This review summarizes the classical physiology of macrophages and polarization, iNOS activities, and evidence towards TAM reprogramming with current information in glioblastoma and melanoma models, and the immunomodulatory and therapeutic options using iNOS or NO-dependent strategies.
Keywords: M1; M2; arginase; cancer progression; iNOS; miRNA; nitric oxide; tumor-associated macrophage.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Kashfi K. Anti-inflammatory agents as cancer therapeutics. Adv. Pharmacol. 2009;57:31–89. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

