Therapeutic Benefit of Galectin-1: Beyond Membrane Repair, a Multifaceted Approach to LGMD2B
- PMID: 34831431
- PMCID: PMC8621416
- DOI: 10.3390/cells10113210
Therapeutic Benefit of Galectin-1: Beyond Membrane Repair, a Multifaceted Approach to LGMD2B
Abstract
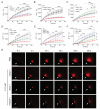
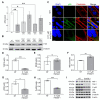
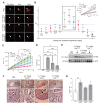
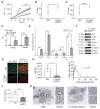
Two of the main pathologies characterizing dysferlinopathies are disrupted muscle membrane repair and chronic inflammation, which lead to symptoms of muscle weakness and wasting. Here, we used recombinant human Galectin-1 (rHsGal-1) as a therapeutic for LGMD2B mouse and human models. Various redox and multimerization states of Gal-1 show that rHsGal-1 is the most effective form in both increasing muscle repair and decreasing inflammation, due to its monomer-dimer equilibrium. Dose-response testing shows an effective 25-fold safety profile between 0.54 and 13.5 mg/kg rHsGal-1 in Bla/J mice. Mice treated weekly with rHsGal-1 showed downregulation of canonical NF-κB inflammation markers, decreased muscle fat deposition, upregulated anti-inflammatory cytokines, increased membrane repair, and increased functional movement compared to non-treated mice. Gal-1 treatment also resulted in a positive self-upregulation loop of increased endogenous Gal-1 expression independent of NF-κB activation. A similar reduction in disease pathologies in patient-derived human cells demonstrates the therapeutic potential of Gal-1 in LGMD2B patients.
Keywords: Galectin-1; LGMD2B; NF-ĸB; cytokines; inflammation; membrane repair; muscular dystrophy.
Conflict of interest statement
Authors of this manuscript have the following declaration of interests: The University of Nevada-Reno has been issued a patent in the U.S. (# US20130065242 A1) and Australia (# 45557BOA/VPB) for “Methods for diagnosing, prognosing and treating muscular dystrophy”. PMVR is an inventor on these patents. Strykagen currently holds the license for this technology. Brigham Young University has a patent for “Galectin-1 immunomodulation and myogenic improvements in muscle diseases and autoimmune disorders.” (#U.S. Pat. No. 62/161,027. PCT/US2021/026232). This does not alter our adherence to
Figures
Similar articles
-
Treatment with galectin-1 improves myogenic potential and membrane repair in dysferlin-deficient models.PLoS One. 2020 Sep 3;15(9):e0238441. doi: 10.1371/journal.pone.0238441. eCollection 2020. PLoS One. 2020. PMID: 32881965 Free PMC article.
-
Evaluating Therapeutic Activity of Galectin-1 in Sarcolemma Repair of Skeletal Muscle.Methods Mol Biol. 2022;2442:663-683. doi: 10.1007/978-1-0716-2055-7_36. Methods Mol Biol. 2022. PMID: 35320552
-
Treatment with Recombinant Human MG53 Protein Increases Membrane Integrity in a Mouse Model of Limb Girdle Muscular Dystrophy 2B.Mol Ther. 2017 Oct 4;25(10):2360-2371. doi: 10.1016/j.ymthe.2017.06.025. Epub 2017 Jul 3. Mol Ther. 2017. PMID: 28750735 Free PMC article.
-
Muscle Cells Fix Breaches by Orchestrating a Membrane Repair Ballet.J Neuromuscul Dis. 2018;5(1):21-28. doi: 10.3233/JND-170251. J Neuromuscul Dis. 2018. PMID: 29480214 Free PMC article. Review.
-
Dysferlin function in skeletal muscle: Possible pathological mechanisms and therapeutical targets in dysferlinopathies.Exp Neurol. 2016 Sep;283(Pt A):246-54. doi: 10.1016/j.expneurol.2016.06.026. Epub 2016 Jun 25. Exp Neurol. 2016. PMID: 27349407 Review.
Cited by
-
Molecular mechanisms and therapeutic strategies for neuromuscular diseases.Cell Mol Life Sci. 2024 Apr 28;81(1):198. doi: 10.1007/s00018-024-05229-9. Cell Mol Life Sci. 2024. PMID: 38678519 Free PMC article. Review.
-
Pharmacotherapeutic Approaches to Treatment of Muscular Dystrophies.Biomolecules. 2023 Oct 17;13(10):1536. doi: 10.3390/biom13101536. Biomolecules. 2023. PMID: 37892218 Free PMC article. Review.
-
Minimal expression of dysferlin prevents development of dysferlinopathy in dysferlin exon 40a knockout mice.Acta Neuropathol Commun. 2023 Jan 18;11(1):15. doi: 10.1186/s40478-022-01473-x. Acta Neuropathol Commun. 2023. PMID: 36653852 Free PMC article.
References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

