A Boron Delivery Antibody (BDA) with Boronated Specific Residues: New Perspectives in Boron Neutron Capture Therapy from an In Silico Investigation
- PMID: 34831449
- PMCID: PMC8618907
- DOI: 10.3390/cells10113225
A Boron Delivery Antibody (BDA) with Boronated Specific Residues: New Perspectives in Boron Neutron Capture Therapy from an In Silico Investigation
Abstract
Boron Neutron Capture Therapy (BNCT) is a tumor cell-selective radiotherapy based on a nuclear reaction that occurs when the isotope boron-10 (10B) is radiated by low-energy thermal neutrons or epithermal neutrons, triggering a nuclear fission response and enabling a selective administration of irradiation to cells. Hence, we need to create novel delivery agents containing 10B with high tumor selectivity, but also exhibiting low intrinsic toxicity, fast clearance from normal tissue and blood, and no pharmaceutical effects. In the past, boronated monoclonal antibodies have been proposed using large boron-containing molecules or dendrimers, but with no investigations in relation to maintaining antibody specificity and structural and functional features. This work aims at improving the potential of monoclonal antibodies applied to BNCT therapy, identifying in silico the best native residues suitable to be substituted with a boronated one, carefully evaluating the effect of boronation on the 3D structure of the monoclonal antibody and on its binding affinity. A boronated monoclonal antibody was thus generated for specific 10B delivery. In this context, we have developed a case study of Boron Delivery Antibody Identification Pipeline, which has been tested on cetuximab. Cetuximab is an epidermal growth factor receptor (EGFR) inhibitor used in the treatment of metastatic colorectal cancer, metastatic non-small cell lung cancer, and head and neck cancer.
Keywords: 4-borono-L-phenylalanine; Boron Delivery Antibody strategy; Boron Neutron Capture Therapy; docking; molecular dynamics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
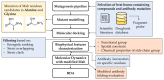
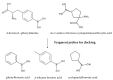
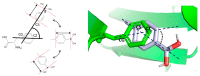
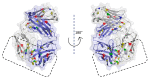
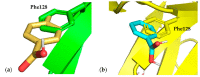

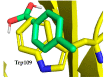
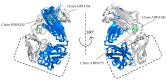
References
-
- Worm D.J., Hoppenz P., Els-Heindl S., Kellert M., Kuhnert R., Saretz S., Köbberling J., Riedl B., Hey-Hawkins E., Beck-Sickinger A.G. Selective Neuropeptide Y Conjugates with Maximized Carborane Loading as Promising Boron Delivery Agents for Boron Neutron Capture Therapy. J. Med. Chem. 2020;63:2358–2371. doi: 10.1021/acs.jmedchem.9b01136. - DOI - PubMed
-
- Sauerwein W.A.G., Bet P.M., Wittig A. In: Neutron Capture Therapy. Wittig A., Moss R., Nakagawa Y., editors. Springer; Berlin/Heidelberg, Germany: 2012. - DOI
-
- Hirose K., Konno A., Hiratsuka J., Yoshimoto S., Kato T., Ono K., Otsuki N., Hatazawa J., Tanaka H., Takayama K., et al. Boron neutron capture therapy using cyclotron-based epithermal neutron source and borofalan (10B) for recurrent or locally advanced head and neck cancer (JHN002): An open-label phase II trial. Radiother Oncol. 2021;155:182–187. doi: 10.1016/j.radonc.2020.11.001. - DOI - PubMed
-
- STELLA PHARMA. [(accessed on 19 October 2021)]. Available online: https://stella-pharma.co.jp/cp-bin/wordpress5/wp-content/uploads/2020/05....
Publication types
MeSH terms
Substances
Grants and funding
- 857650/EU project EOSC-Pillar
- BBMRI/pan-European research infrastructure for Biobanking and BioMolecular Re-sources Research In-frastructure (BBMRI)
- EGI-ACE/EGI-Advanced Computing for Eosc
- RF-2019-12370396/Progetti ordinari di ricerca finalizzata, Ministero della salute
- PON R&I PIR01_00017/CENTRO NAZIONALE DI RICERCA IN BIOINFORMATICA PER LE SCIENZE "OMICHE" CNRBIOMICS
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

