Activated Carbon from Palm Date Seeds for CO2 Capture
- PMID: 34831898
- PMCID: PMC8624853
- DOI: 10.3390/ijerph182212142
Activated Carbon from Palm Date Seeds for CO2 Capture
Abstract
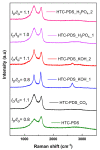
The process of carbon dioxide capture and storage is seen as a critical strategy to mitigate the so-called greenhouse effect and the planetary climate changes associated with it. In this study, we investigated the CO2 adsorption capacity of various microporous carbon materials originating from palm date seeds (PDS) using green chemistry synthesis. The PDS was used as a precursor for the hydrochar and activated carbon (AC). Typically, by using the hydrothermal carbonization (HTC) process, we obtained a powder that was then subjected to an activation step using KOH, H3PO4 or CO2, thereby producing the activated HTC-PDS samples. Beyond their morphological and textural characteristics, we investigated the chemical composition and lattice ordering. Most PDS-derived powders have a high surface area (>1000 m2 g-1) and large micropore volume (>0.5 cm3 g-1). However, the defining characteristic for the maximal CO2 uptake (5.44 mmol g-1, by one of the alkaline activated samples) was the lattice restructuring that occurred. This work highlights the need to conduct structural and elemental analysis of carbon powders used as gas adsorbents and activated with chemicals that can produce graphite intercalation compounds.
Keywords: CO2 capture; activation; adsorption; hydrothermal carbonization; palm date seeds.
Conflict of interest statement
The authors declare no conflict of interest. The author Sabina Alexandra Nicolae is an employee of MDPI, however, she does not work for the journal IJERPH at the time of submission and publication.
Figures



References
-
- Siriwardane R.V., Shen M.-S., Fisher E.P., Poston J.A. Adsorption of CO2 on Molecular Sieves and Activated Carbon. Energy Fuels. 2001;15:279–284. doi: 10.1021/ef000241s. - DOI
-
- Jadhav P.D., Chatti R.V., Biniwale R.B., Labhsetwar N.K., Devotta S., Rayalu S.S. Monoethanol Amine Modified Zeolite 13X for CO2 Adsorption at Different Temperatures. Energy Fuels. 2007;21:3555–3559. doi: 10.1021/ef070038y. - DOI
-
- Gray M.L., Soong Y., Champagne K.J., Pennline H., Baltrus J.P., Stevens R.W., Khatri R., Chuang S.S.C., Filburn T. Improved immobilized carbon dioxide capture sorbents. Fuel Process. Technol. 2005;86:1449–1455. doi: 10.1016/j.fuproc.2005.01.005. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

