In Vivo Secretion of β-Lactamase-Carrying Outer Membrane Vesicles as a Mechanism of β-Lactam Therapy Failure
- PMID: 34832035
- PMCID: PMC8625792
- DOI: 10.3390/membranes11110806
In Vivo Secretion of β-Lactamase-Carrying Outer Membrane Vesicles as a Mechanism of β-Lactam Therapy Failure
Abstract
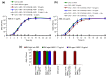
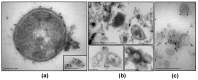
Outer membrane vesicles carrying β-lactamase (βLOMVs) protect bacteria against β-lactam antibiotics under experimental conditions, but their protective role during a patient's treatment leading to the therapy failure is unknown. We investigated the role of βLOMVs in amoxicillin therapy failure in a patient with group A Streptococcus pyogenes (GAS) pharyngotonsillitis. The patient's throat culture was examined by standard microbiological procedures. Bacterial vesicles were analyzed for β-lactamase by immunoblot and the nitrocefin assay, and in vivo secretion of βLOMVs was detected by electron microscopy. These analyses demonstrated that the patient's throat culture grew, besides amoxicillin-susceptible GAS, an amoxicillin-resistant nontypeable Haemophilus influenzae (NTHi), which secreted βLOMVs. Secretion and β-lactamase activity of NTHi βLOMVs were induced by amoxicillin concentrations reached in the tonsils during therapy. The presence of NTHi βLOMVs significantly increased the minimal inhibitory concentration of amoxicillin for GAS and thereby protected GAS against bactericidal concentrations of amoxicillin. NTHi βLOMVs were identified in the patient's pharyngotonsillar swabs and saliva, demonstrating their secretion in vivo at the site of infection. We conclude that the pathogen protection via βLOMVs secreted by the flora colonizing the infection site represents a yet underestimated mechanism of β-lactam therapy failure that warrants attention in clinical studies.
Keywords: GAS protection; Haemophilus influenzae; amoxicillin therapy failure; group A Streptococcus pyogenes (GAS); in vivo secretion; pharyngotonsillitis; β-lactamase-carrying outer membrane vesicles.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no roles in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

References
-
- Shulman S.T., Bisno A.L., Clegg H.W., Gerber M.A., Kaplan E.L., Lee G., Martin J.M., Van Beneden C. Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2012;55:e86–e102. doi: 10.1093/cid/cis629. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

