Neurosurgical Approaches to Brain Tissue Harvesting for the Establishment of Cell Cultures in Neural Experimental Cell Models
- PMID: 34832259
- PMCID: PMC8624371
- DOI: 10.3390/ma14226857
Neurosurgical Approaches to Brain Tissue Harvesting for the Establishment of Cell Cultures in Neural Experimental Cell Models
Abstract
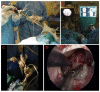
In recent decades, cell biology has made rapid progress. Cell isolation and cultivation techniques, supported by modern laboratory procedures and experimental capabilities, provide a wide range of opportunities for in vitro research to study physiological and pathophysiological processes in health and disease. They can also be used very efficiently for the analysis of biomaterials. Before a new biomaterial is ready for implantation into tissues and widespread use in clinical practice, it must be extensively tested. Experimental cell models, which are a suitable testing ground and the first line of empirical exploration of new biomaterials, must contain suitable cells that form the basis of biomaterial testing. To isolate a stable and suitable cell culture, many steps are required. The first and one of the most important steps is the collection of donor tissue, usually during a surgical procedure. Thus, the collection is the foundation for the success of cell isolation. This article explains the sources and neurosurgical procedures for obtaining brain tissue samples for cell isolation techniques, which are essential for biomaterial testing procedures.
Keywords: biomaterial testing; brain; cell isolation; experimental cell models; neurosurgery.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Keller J.M., Frega M. Vitro Neuronal Networks. Springer Nature; Basingstoke, UK: 2019. Past, present, and future of neuronal models in vitro; pp. 3–17. - PubMed
-
- Trope C., Sigurdsson K. Use of tissue culture in predictive testing of drug sensitivity in human ovarian cancer. Correlation between in vitro results and the response in vivo. Neoplasma. 1982;29:309–314. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

