Optical Properties of Transparent Rare-Earth Doped Sol-Gel Derived Nano-Glass Ceramics
- PMID: 34832273
- PMCID: PMC8623871
- DOI: 10.3390/ma14226871
Optical Properties of Transparent Rare-Earth Doped Sol-Gel Derived Nano-Glass Ceramics
Abstract
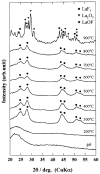
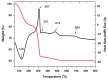
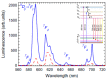
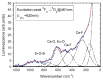
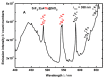
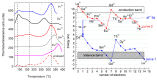
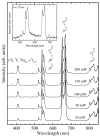
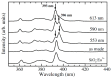
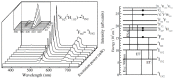
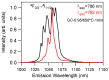
Rare-earth doped oxyfluoride glass ceramics represent a new generation of tailorable optical materials with high potential for optical-related applications such as optical amplifiers, optical waveguides, and white LEDs. Their key features are related to the high transparency and remarkable luminescence properties, while keeping the thermal and chemical advantages of oxide glasses. Sol-gel chemistry offers a flexible synthesis approach with several advantages, such as lower processing temperature, the ability to control the purity and homogeneity of the final materials on a molecular level, and the large compositional flexibility. The review will be focused on optical properties of sol-gel derived nano-glass ceramics related to the RE-doped luminescent nanocrystals (fluorides, chlorides, oxychlorides, etc.) such as photoluminescence, up-conversion luminescence, thermoluminescence and how these properties are influenced by their specific processing, mostly focusing on the findings from our group and similar ones in the literature, along with a discussion of perspectives, potential challenges, and future development directions.
Keywords: fluorides; glass ceramic; luminescence; nanocrystals; rare-earth; sol-gel.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Deubener J., Allix M., Davis M.J., Duran A., Höche T., Honma T., Komatsu T., Krüger S., Mitra I., Müller R., et al. Updated definition of glass-ceramics. J. Non-Cryst. Solids. 2018;501:3–10. doi: 10.1016/j.jnoncrysol.2018.01.033. - DOI
-
- Pablos-Martin A., Duran A., Pascual M.J. Nanocrystallisation in oxyfluoride systems: Mechanisms of crystallization and photonic properties. Int. Mater. Rev. 2012;57:165–186. doi: 10.1179/1743280411Y.0000000004. - DOI
-
- Brinker C.J., Scherer G. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing. 1st ed. Academic Press Inc.; New York, NY, USA: 1990. Levy, D.; Zayat, M. The Sol-Gel Handbook: Synthesis, Characterization and Applications; Wiley-VCH: Weinheim, Germany, 2015.
-
- Fujihara S., Mochizuki C., Kimura T. Formation of LaF3 microcrystals in sol-gel silica. J. Non-Cryst. Solids. 1999;244:267–274. doi: 10.1016/S0022-3093(99)00009-5. - DOI
Publication types
LinkOut - more resources
Full Text Sources

