Co-Existence of Iron Oxide Nanoparticles and Manganese Oxide Nanorods as Decoration of Hollow Carbon Spheres for Boosting Electrochemical Performance of Li-Ion Battery
- PMID: 34832303
- PMCID: PMC8620810
- DOI: 10.3390/ma14226902
Co-Existence of Iron Oxide Nanoparticles and Manganese Oxide Nanorods as Decoration of Hollow Carbon Spheres for Boosting Electrochemical Performance of Li-Ion Battery
Abstract
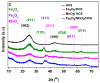
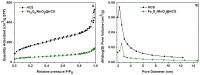
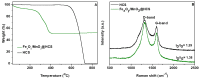
Here, we report that mesoporous hollow carbon spheres (HCS) can be simultaneously functionalized: (i) endohedrally by iron oxide nanoparticle and (ii) egzohedrally by manganese oxide nanorods (FexOy/MnO2/HCS). Detailed analysis reveals a high degree of graphitization of HCS structures. The mesoporous nature of carbon is further confirmed by N2 sorption/desorption and transmission electron microscopy (TEM) studies. The fabricated molecular heterostructure was tested as the anode material of a lithium-ion battery (LIB). For both metal oxides under study, their mixture stored in HCS yielded a significant increase in electrochemical performance. Its electrochemical response was compared to the HCS decorated with a single component of the respective metal oxide applied as a LIB electrode. The discharge capacity of FexOy/MnO2/HCS is 1091 mAhg-1 at 5 Ag-1, and the corresponding coulombic efficiency (CE) is as high as 98%. Therefore, the addition of MnO2 in the form of nanorods allows for boosting the nanocomposite electrochemical performance with respect to the spherical nanoparticles due to better reversible capacity and cycling performance. Thus, the structure has great potential application in the LIB field.
Keywords: battery; carbon spheres; metal oxide nanoparticles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Synthesis of One-Dimensional Mesoporous Ag Nanoparticles-Modified TiO2 Nanofibers by Electrospinning for Lithium Ion Batteries.Materials (Basel). 2019 Aug 18;12(16):2630. doi: 10.3390/ma12162630. Materials (Basel). 2019. PMID: 31426615 Free PMC article.
-
A hierarchical hybrid design for high performance tin based Li-ion battery anodes.Nanotechnology. 2013 May 24;24(20):205401. doi: 10.1088/0957-4484/24/20/205401. Epub 2013 Apr 19. Nanotechnology. 2013. PMID: 23598519
-
Mesoporous Silicon Hollow Nanocubes Derived from Metal-Organic Framework Template for Advanced Lithium-Ion Battery Anode.ACS Nano. 2017 May 23;11(5):4808-4815. doi: 10.1021/acsnano.7b01185. Epub 2017 May 8. ACS Nano. 2017. PMID: 28467837
-
Filled Carbon Nanotubes as Anode Materials for Lithium-Ion Batteries.Molecules. 2020 Feb 27;25(5):1064. doi: 10.3390/molecules25051064. Molecules. 2020. PMID: 32120977 Free PMC article. Review.
-
A Review on Recent Advancements of Ni-NiO Nanocomposite as an Anode for High-Performance Lithium-Ion Battery.Nanomaterials (Basel). 2022 Aug 25;12(17):2930. doi: 10.3390/nano12172930. Nanomaterials (Basel). 2022. PMID: 36079968 Free PMC article. Review.
Cited by
-
Organic-Inorganic Hybrid Interfaces Enable the Preparation of Nitrogen-Doped Hollow Carbon Nanospheres as High-Performance Anodes for Lithium and Potassium-Ion Batteries.Materials (Basel). 2023 Jul 11;16(14):4936. doi: 10.3390/ma16144936. Materials (Basel). 2023. PMID: 37512212 Free PMC article.
References
-
- Amjad S., Rudramoorthy R., Neelakrishnan S., Varman K.S.R., Arjunan T., Shaik A. Evaluation of energy requirements for all-electric range of plug-in hybrid electric two-wheeler. Energy. 2011;36:1623–1629. doi: 10.1016/j.energy.2010.12.069. - DOI
-
- Wang J., Zhao H., Zeng Z., Lv P., Li Z., Zhang T., Yang T. Nano-sized Fe3O4/carbon as anode material for lithium ion battery. Mater. Chem. Phys. 2014;148:699–704. doi: 10.1016/j.matchemphys.2014.08.037. - DOI
LinkOut - more resources
Full Text Sources

