Experimental Induction of Tenacibaculosis in Atlantic Salmon (Salmo salar L.) Using Tenacibaculum maritimum, T. dicentrarchi, and T. finnmarkense
- PMID: 34832595
- PMCID: PMC8623880
- DOI: 10.3390/pathogens10111439
Experimental Induction of Tenacibaculosis in Atlantic Salmon (Salmo salar L.) Using Tenacibaculum maritimum, T. dicentrarchi, and T. finnmarkense
Abstract
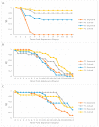
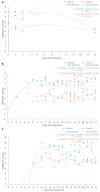
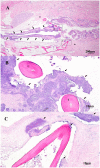
There is a limited understanding of the pathogenesis of tenacibaculosis in Atlantic salmon (Salmo salar L.) and there are few reproducible exposure models for comparison. Atlantic salmon were exposed via bath to Tenacibaculum maritimum, T. dicentrarchi, or T. finnmarkense, and were then grouped with naïve cohabitants. Mortalities had exaggerated clinical signs of mouthrot, a presentation of tenacibaculosis characterized by epidermal ulceration and yellow plaques, on the mouth and less frequently on other tissues. Histopathology showed tissue spongiosis, erosion, ulceration, and necrosis ranging from mild to marked, locally to regionally extensive with mats of intralesional bacteria on the rostrum, vomer, gill rakers, gill filaments, and body surface. Exposure to T. maritimum resulted in less than a 0.4 probability of survival for both exposed and cohabitants until Day 21. Exposures to T. dicentrarchi resulted in 0 and 0.55 (exposed), and 0.8 and 0.9 (cohabitant) probability of survival to Day 12 post-exposure, while T. finnmarkense had a 0.9 probability of survival to Day 12 for all groups. This experimental infection model will be useful to further investigate the pathogenesis of tenacibaculosis, its treatment, and immunity to Tenacibaculum species.
Keywords: aquaculture; bath-exposure; dysbiosis; histopathology; mouthrot; qPCR; tenacibaculosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources

