Characterisation of Macrophage Polarisation in Mice Infected with Ninoa Strain of Trypanosoma cruzi
- PMID: 34832600
- PMCID: PMC8622189
- DOI: 10.3390/pathogens10111444
Characterisation of Macrophage Polarisation in Mice Infected with Ninoa Strain of Trypanosoma cruzi
Abstract
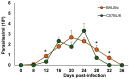
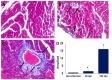
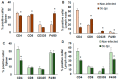
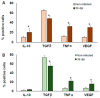
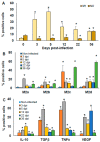
Macrophages (MΦ) play a key role in the development of the protective immune response against Trypanosoma cruzi infection. To determine the role of MΦ subtypes M1 and M2 in the development of immunity against the Mexican strain of T. cruzi (Ninoa strain), we have analysed in a time course the infection and characterised the M1 and M2 subtypes in two mouse models, BALB/c and C57BL/6. After infection, BALB/c mice developed an increased blood parasite load and the parasites were cleared from the blood one week later than in C57BL/6 mice. However, similar cellular infiltrate and cardiac alterations were observed between BALB/c and C57BL/6 mice. At 36 days, the T. cruzi infection differentially modulated the expression of immune cells, and both the BALB/c and C57BL/6 mice significantly reduced TCD4+ cells. However, BALB/c mice produced significantly more TCD8+ than C57BL/6 mice in the spleen and lymph nodes. Furthermore, BALB/c mice produce significantly more MΦ in the spleen, while C57BL/6 produce similar levels to uninfected mice. The M1 MΦ ratio increased significantly at 3-5 days post-infection (dpi), but then decreased slightly. On the contrary, the M2 MΦ were low at the beginning of the infection, but the proportion of M1 and M2 MΦ at 36 dpi was similar. Importantly, the MΦ subtypes M2c and M2d significantly increased the induction of tissue repair by the end of the acute phase of the infection. These results indicate that the Ninoa strain has developed strategies to modulate the immune response, with fine differences depending on the genetic background of the host.
Keywords: BALB/c mice; C57BL/6 mice; Trypanosoma cruzi; chagas disease; macrophage polarisation; ninoa strain; parasitic burden.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Mena-Marín A.L., Zeledón R., Morales J.A., Pereira M., Urbina A. Sex influence on the susceptibility of Swiss mice to Trypanosoma cruzi [Influencia del sexo en la susceptibilidad de ratones Swiss a Trypanosoma cruzi] Boletín de Malariogía y Salud Ambiental. 2012;LII:233–244.
-
- PAHO/WHO Pan American Health Organization. Chagas Disease. [(accessed on 6 May 2021)]. Available online: https://www.paho.org/en/topics/chagas-disease.
-
- WHO World Health Organization. Chagas Disease (American Trypanosomiasis) [(accessed on 6 May 2021)]. Available online: https://www.who.int/health-topics/chagas-disease#tab=tab_1.
-
- Bern C., Martin D.L., Gilman R.H. Acute and congenital Chagas disease. Adv. Parasitol. 2011;75:19–47. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

