The Global Emergence of Human Babesiosis
- PMID: 34832603
- PMCID: PMC8623124
- DOI: 10.3390/pathogens10111447
The Global Emergence of Human Babesiosis
Erratum in
-
Correction: Kumar et al. The Global Emergence of Human Babesiosis. Pathogens 2021, 10, 1447.Pathogens. 2022 May 23;11(5):607. doi: 10.3390/pathogens11050607. Pathogens. 2022. PMID: 35631130 Free PMC article.
-
Correction: Kumar et al. The Global Emergence of Human Babesiosis. Pathogens 2021, 10, 1447.Pathogens. 2022 Aug 17;11(8):923. doi: 10.3390/pathogens11080923. Pathogens. 2022. PMID: 36015072 Free PMC article.
Abstract
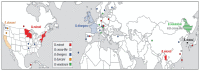
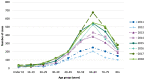
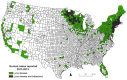
Babesiosis is an emerging tick-borne disease caused by intraerythrocytic protozoa that are primarily transmitted by hard-bodied (ixodid) ticks and rarely through blood transfusion, perinatally, and organ transplantation. More than 100 Babesia species infect a wide spectrum of wild and domestic animals worldwide and six have been identified as human pathogens. Babesia microti is the predominant species that infects humans, is found throughout the world, and causes endemic disease in the United States and China. Babesia venatorum and Babesia crassa-like agent also cause endemic disease in China. Babesia divergens is the predominant species in Europe where fulminant cases have been reported sporadically. The number of B. microti infections has been increasing globally in recent decades. In the United States, more than 2000 cases are reported each year, although the actual number is thought to be much higher. In this review of the epidemiology of human babesiosis, we discuss epidemiologic tools used to monitor disease location and frequency; demographics and modes of transmission; the location of human babesiosis; the causative Babesia species in the Americas, Europe, Asia, Africa, and Australia; the primary clinical characteristics associated with each of these infections; and the increasing global health burden of this disease.
Keywords: Babesia microti; babesiosis; case surveillance; epidemiology; immunoepidemiology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

