Microneedles in Drug Delivery: Progress and Challenges
- PMID: 34832733
- PMCID: PMC8623547
- DOI: 10.3390/mi12111321
Microneedles in Drug Delivery: Progress and Challenges
Abstract
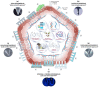
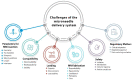
In recent years, an innovative transdermal delivery technology has attracted great interest for its ability to distribute therapeutics and cosmeceuticals for several applications, including vaccines, drugs, and biomolecules for skin-related problems. The advantages of microneedle patch technology have been extensively evaluated in the latest literature; hence, the academic publications in this area are rising exponentially. Like all new technologies, the microneedle patch application has great potential but is not without limitations. In this review, we will discuss the possible limitations by highlighting the areas where a great deal of improvements are required. Emphasising these concerns early on should help scientists and technologists to address the matters in a timely fashion and to use their resources wisely.
Keywords: microneedles; transdermal drug delivery; transdermal patch technology; vaccine delivery.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources

