Electroreforming of Biomass for Value-Added Products
- PMID: 34832816
- PMCID: PMC8619709
- DOI: 10.3390/mi12111405
Electroreforming of Biomass for Value-Added Products
Abstract
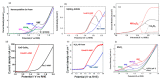
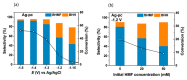
Humanity's overreliance on fossil fuels for chemical and energy production has resulted in uncontrollable carbon emissions that have warranted widespread concern regarding global warming. To address this issue, there is a growing body of research on renewable resources such as biomass, of which cellulose is the most abundant type. In particular, the electrochemical reforming of biomass is especially promising, as it allows greater control over valorization processes and requires milder conditions. Driven by renewable electricity, electroreforming of biomass can be green and sustainable. Moreover, green hydrogen generation can be coupled to anodic biomass electroforming, which has attracted ever-increasing attention. The following review is a summary of recent developments related to electroreforming cellulose and its derivatives (glucose, hydroxymethylfurfural, levulinic acid). The electroreforming of biomass can be achieved on the anode of an electrochemical cell through electrooxidation, as well as on the cathode through electroreduction. Recent advances in the anodic electroreforming of cellulose and cellulose-derived glucose and 5-hydrooxylmethoylfurural (5-HMF) are first summarized. Then, the key achievements in the cathodic electroreforming of cellulose and cellulose-derived 5-HMF and levulinic acid are discussed. Afterward, the emerging research focusing on coupling hydrogen evolution with anodic biomass reforming for the cogeneration of green hydrogen fuel and value-added chemicals is reviewed. The final chapter of this paper provides our perspective on the challenges and future research directions of biomass electroreforming.
Keywords: biomass electroreforming; cellulose; electrochemical hydrogenation; electrooxidation; green hydrogen.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
























Similar articles
-
Electroreforming of plastic wastes for value-added products.Chem Commun (Camb). 2024 Dec 17;61(1):33-45. doi: 10.1039/d4cc04574b. Chem Commun (Camb). 2024. PMID: 39601665 Review.
-
Raw biomass electroreforming coupled to green hydrogen generation.Nat Commun. 2021 Mar 31;12(1):2008. doi: 10.1038/s41467-021-22250-9. Nat Commun. 2021. PMID: 33790295 Free PMC article.
-
Photocatalytic nanomaterials and their implications towards biomass conversion for renewable chemical and fuel production.Nanoscale Adv. 2024 Sep 30;6(21):5258-84. doi: 10.1039/d4na00447g. Online ahead of print. Nanoscale Adv. 2024. PMID: 39359352 Free PMC article. Review.
-
Paired electrocatalysis in 5-hydroxymethylfurfural valorization.Front Chem. 2022 Oct 21;10:1055865. doi: 10.3389/fchem.2022.1055865. eCollection 2022. Front Chem. 2022. PMID: 36339046 Free PMC article. Review.
-
Polymerization of nonfood biomass-derived monomers to sustainable polymers.Top Curr Chem. 2014;353:185-227. doi: 10.1007/128_2014_539. Top Curr Chem. 2014. PMID: 24699900 Review.
Cited by
-
Electrochemical recycling of polymeric materials.Chem Sci. 2024 May 21;15(23):8606-8624. doi: 10.1039/d4sc01754d. eCollection 2024 Jun 12. Chem Sci. 2024. PMID: 38873080 Free PMC article. Review.
-
Studying the Competition between Glucose Oxidation and Oxygen Evolution Reaction: Toward a Membrane-Free Electrolyzer for the Production of H2 and Added Value Products.ACS Sustain Chem Eng. 2025 Mar 5;13(13):4963-4974. doi: 10.1021/acssuschemeng.4c09249. eCollection 2025 Apr 7. ACS Sustain Chem Eng. 2025. PMID: 40212581 Free PMC article.
References
-
- IPCC Global warming of 1.5 °C. An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change. IPCC Sr15, October 2, pp. 17–20. 2018. [(accessed on 11 August 2021)]. Available online: www.environmentalgraphiti.org.
-
- IPCC . Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press; Cambridge, UK: 2021.
-
- Jevrejeva S., Jackson L.P., Grinsted A., Lincke D., Marzeion B. Flood damage costs under the sea level rise with warming of 1.5 °C and 2 °C. Environ. Res. Lett. 2018;13:074014. doi: 10.1088/1748-9326/aacc76. - DOI
-
- Deryng D., Conway D., Ramankutty N., Price J., Warren R. Global crop yield response to extreme heat stress under multiple climate change futures. Environ. Res. Lett. 2014;9:034011. doi: 10.1088/1748-9326/9/3/034011. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

