Diversity-Oriented Synthesis: Amino Acetophenones as Building Blocks for the Synthesis of Natural Product Analogs
- PMID: 34832909
- PMCID: PMC8619038
- DOI: 10.3390/ph14111127
Diversity-Oriented Synthesis: Amino Acetophenones as Building Blocks for the Synthesis of Natural Product Analogs
Abstract
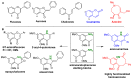
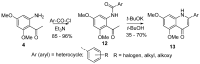
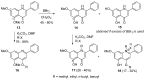
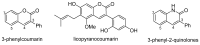
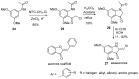
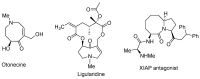
Diversity-Oriented Synthesis (DOS) represents a strategy to obtain molecule libraries with diverse structural features starting from one common compound in limited steps of synthesis. During the last two decades, DOS has become an unmissable strategy in organic synthesis and is fully integrated in various drug discovery processes. On the other hand, natural products with multiple relevant pharmacological properties have been extensively investigated as scaffolds for ligand-based drug design. In this article, we report the amino dimethoxyacetophenones that can be easily synthesized and scaled up from the commercially available 3,5-dimethoxyaniline as valuable starting blocks for the DOS of natural product analogs. More focus is placed on the synthesis of analogs of flavones, coumarins, azocanes, chalcones, and aurones, which are frequently studied as lead compounds in drug discovery.
Keywords: Diversity-Oriented Synthesis; amino acetophenones; aurones; azocanes; coumarins; flavones; natural products; quinolones.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





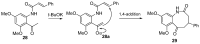
References
Publication types
LinkOut - more resources
Full Text Sources

