Clinical Characteristics of Mitochondrial Encephalomyopathy, Lactic Acidosis, and Stroke-Like Episodes
- PMID: 34832987
- PMCID: PMC8617702
- DOI: 10.3390/life11111111
Clinical Characteristics of Mitochondrial Encephalomyopathy, Lactic Acidosis, and Stroke-Like Episodes
Abstract
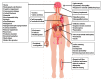
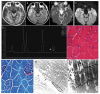
Mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes (MELAS) syndrome, a maternally inherited mitochondrial disorder, is characterized by its genetic, biochemical and clinical complexity. The most common mutation associated with MELAS syndrome is the mtDNA A3243G mutation in the MT-TL1 gene encoding the mitochondrial tRNA-leu(UUR), which results in impaired mitochondrial translation and protein synthesis involving the mitochondrial electron transport chain complex subunits, leading to impaired mitochondrial energy production. Angiopathy, either alone or in combination with nitric oxide (NO) deficiency, further contributes to multi-organ involvement in MELAS syndrome. Management for MELAS syndrome is amostly symptomatic multidisciplinary approach. In this article, we review the clinical presentations, pathogenic mechanisms and options for management of MELAS syndrome.
Keywords: MELAS; genetics; mitochondrial DNA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources

