Distinctive Role of the Systemic Inflammatory Profile in Non-Small-Cell Lung Cancer Younger and Elderly Patients Treated with a PD-1 Immune Checkpoint Blockade: A Real-World Retrospective Multi-Institutional Analysis
- PMID: 34833111
- PMCID: PMC8621400
- DOI: 10.3390/life11111235
Distinctive Role of the Systemic Inflammatory Profile in Non-Small-Cell Lung Cancer Younger and Elderly Patients Treated with a PD-1 Immune Checkpoint Blockade: A Real-World Retrospective Multi-Institutional Analysis
Abstract
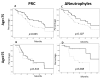
An immune checkpoint blockade with mAbs to PD-1 and PD-L1 is an expanding therapeutic option for mNSCLC patients. This treatment strategy is based on the use of mAbs able to restore the anti-tumor activity of intratumoral T cells inhibited by PD-1 binding to PD-L1/2 on tumor and inflammatory cells. It has been speculated that a chronic status of systemic inflammation as well as the immunosenescence physiologically occurring in elderly patients may affect the efficacy of the treatment and the occurrence of irAEs. We performed a multi-institutional retrospective study aimed at evaluating the effects of these mAbs (nivolumab or atezolizumab) in 117 mNSCLC patients younger (90 cases) and older (27 cases) than 75 years in correlation with multiple inflammatory parameters (NLR, CRP, ESR, LDH and PCT). No differences were observed when the cohorts were compared in terms of the frequency of PFS, OS, inflammatory markers and immune-related adverse events (irAEs). Similarly, the occurrence of irAEs was strictly correlated with a prolonged OS survival in both groups. On the contrary, a negative correlation between the high baseline levels of inflammatory markers and OS could be demonstrated in the younger cohort only. Overall, PD-1/PD-L1-blocking mAbs were equally effective in young and elderly mNSCLC patients; however, the detrimental influence of a systemic inflammation at the baseline was only observed in young patients, suggesting different aging-related inflammation immunoregulative effects.
Keywords: age; immune checkpoint blockade; immunosenescence; immunotherapy; inflammatory markers; metastatic non-small-cell lung cancer; real-world evidence study.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Naidoo J., Wang X., Woo K.M., Iyriboz T., Halpenny D., Cunningham J., Chaft J.E., Segal N.H., Callahan M.K., Lesokhin A.M., et al. Pneumonitis in Patients Treated With Anti-Programmed Death-1/Programmed Death Ligand 1 Therapy. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017;35:709–717. doi: 10.1200/JCO.2016.68.2005. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

