Master Protocols for Precision Medicine in Oncology: Overcoming Methodology of Randomized Clinical Trials
- PMID: 34833129
- PMCID: PMC8618758
- DOI: 10.3390/life11111253
Master Protocols for Precision Medicine in Oncology: Overcoming Methodology of Randomized Clinical Trials
Abstract
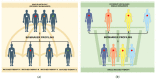
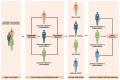
Randomized clinical trials are considered the milestones of clinical research in oncology, and guided the development and approval of new compounds so far. In the last few years, however, molecular and genomic profiling led to a change of paradigm in therapeutic algorithms of many cancer types, with the spread of different biomarker-driven therapies (or targeted therapies). This scenario of "personalized medicine" revolutionized therapeutic strategies and the methodology of the supporting clinical research. New clinical trial designs are emerging to answer to the unmet clinical needs related to the development of these targeted therapies, overcoming the "classical" structure of randomized studies. Innovative trial designs able to evaluate more than one treatment in the same group of patients or many groups of patients with the same treatment (or both) are emerging as a possible future standard in clinical trial methodology. These are identified as "master protocols", and include umbrella, basket and platform trials. In this review, we described the main characteristics of these new trial designs, focusing on the opportunities and limitations of their use in the era of personalized medicine.
Keywords: clinical trials; individualized treatment; methodology; personalized medicine.
Conflict of interest statement
R.D.: personal fees from Astellas, outside the submitted work; M.C.P.: personal fees from Daichii-Sankyo, personal fees from GSK, personal fees from MSD, grants from Roche, grants and personal fees from AstraZeneca and non-financial support from Bayer outside the submitted work; A.G.: non-financial support from Pfizer, outside the submitted work; F.P.: personal fees from Bayer, personal fees from Ipsen, personal fees from Astra Zeneca, personal fees from Bristol Myers Squibb, personal fees from Sandoz, personal fees from Incyte, personal fees from Celgene, personal fees from Pierre Fabre and personal fees from Janssen-Cilag outside the submitted work. The other authors do not declare any conflicts of interest.
Figures
References
-
- Chen J.J., Lu T.-P., Chen D.-T., Wang S.-J. Biomarker adaptive designs in clinical trials. Transl. Cancer Res. 2014;3:279–292.
-
- Pascarella G., Capasso A., Nardone A., Triassi M., Pignata S., Arenare L., Ascierto P., Curvietto M., Maiolino P., D’Aniello R., et al. Costs of clinical trials with anticancer biological agents in an Oncologic Italian Cancer Center using the activity-based costing methodology. PLoS ONE. 2019;14:e0210330. doi: 10.1371/journal.pone.0210330. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources