Bone Marrow Aspirate Concentrate versus Platelet Rich Plasma or Hyaluronic Acid for the Treatment of Knee Osteoarthritis
- PMID: 34833411
- PMCID: PMC8623697
- DOI: 10.3390/medicina57111193
Bone Marrow Aspirate Concentrate versus Platelet Rich Plasma or Hyaluronic Acid for the Treatment of Knee Osteoarthritis
Abstract
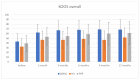
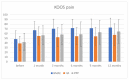
Background: In the last decade, regenerative therapies have become one of the leading disease modifying options for treatment of knee osteoarthritis (OA). Still, there is a lack of trials with a direct comparison of different biological treatments. Our aim was to directly compare clinical outcomes of knee injections of Bone Marrow Aspirate Concentrate (BMAC), Platelet-rich Plasma (PRP), or Hyaluronic acid (HA) in the OA treatment. Methods: Patients with knee pain and osteoarthritis KL grade II to IV were randomized to receive a BMAC, PRP, and HA injection in the knee. VAS, WOMAC, KOOS, and IKDC scores were used to establish baseline values at 1, 3, 6, 9, and 12 months. All side effects were reported. Results: A total of 175 patients with a knee osteoarthritis KL grade II-IV were randomized; 111 were treated with BMAC injection, 30 with HA injection, and 34 patients with PRP injection. There were no differences between these groups when considering KL grade, BMI, age, or gender. There were no serious side effects. The mean VAS scores after 3, 7, 14, and 21 days showed significant differences between groups with a drop of VAS in all groups but with a difference in the BMAC group in comparison to other groups (p < 0.001). There were high statistically significant differences between baseline scores and those after 12 months (p < 0.001) in WOMAC, KOOS, KOOS pain, and IKDC scores, and in addition, there were differences between these scores in the BMAC group in comparison with other groups, except for the PRP group in WOMAC and the partial IKDC score. There were no differences between the HA and PRP groups, although PRP showed a higher level of clinical improvement. Conclusions: Bone marrow aspirate concentrate, Leukocyte rich Platelet Rich Plasma, and Hyaluronic acid injections are safe therapeutic options for knee OA and provide positive clinical outcomes after 12 months in comparison with findings preceding the intervention. BMAC could be better in terms of clinical improvements in the treatment of knee OA than PRP and HA up to 12 months. PRP provides better outcomes than HA during the observation period, but these results are not statistically significant. More randomized controlled trials and high quality comparative studies are needed for direct correlative conclusions.
Keywords: bone marrow aspirate concentrate; hyaluronic acid; knee osteoarthritis; platelet rich plasma; regenerative medicine; stem cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Patients With Knee Osteoarthritis Who Receive Platelet-Rich Plasma or Bone Marrow Aspirate Concentrate Injections Have Better Outcomes Than Patients Who Receive Hyaluronic Acid: Systematic Review and Meta-analysis.Arthroscopy. 2023 Jul;39(7):1714-1734. doi: 10.1016/j.arthro.2023.03.001. Epub 2023 Mar 11. Arthroscopy. 2023. PMID: 36913992
-
Bone marrow aspirate concentrate versus platelet-rich plasma for treating knee osteoarthritis: a one-year non-randomized retrospective comparative study.BMC Musculoskelet Disord. 2022 Jan 3;23(1):23. doi: 10.1186/s12891-021-04910-5. BMC Musculoskelet Disord. 2022. PMID: 34980045 Free PMC article.
-
Platelet-Rich Plasma Versus Hyaluronic Acid for Knee Osteoarthritis: A Systematic Review and Meta-analysis of Randomized Controlled Trials.Am J Sports Med. 2021 Jan;49(1):249-260. doi: 10.1177/0363546520909397. Epub 2020 Apr 17. Am J Sports Med. 2021. PMID: 32302218
-
Bone Marrow Aspirate Concentrate Is Equivalent to Platelet-Rich Plasma for the Treatment of Knee Osteoarthritis at 2 Years: A Prospective Randomized Trial.Am J Sports Med. 2022 Mar;50(3):618-629. doi: 10.1177/03635465211072554. Am J Sports Med. 2022. PMID: 35289231 Clinical Trial.
-
Bone marrow aspirate concentrate injections provide similar results versus viscosupplementation up to 24 months of follow-up in patients with symptomatic knee osteoarthritis. A randomized controlled trial.Knee Surg Sports Traumatol Arthrosc. 2022 Dec;30(12):3958-3967. doi: 10.1007/s00167-021-06793-4. Epub 2021 Nov 12. Knee Surg Sports Traumatol Arthrosc. 2022. PMID: 34767030 Clinical Trial.
Cited by
-
Comparison of Pain and Complications between Outpatients and Inpatients Treated with Bone Marrow Aspirate Concentrate for Knee Osteoarthritis.J Pers Med. 2024 Sep 5;14(9):942. doi: 10.3390/jpm14090942. J Pers Med. 2024. PMID: 39338196 Free PMC article.
-
Mesenchymal stem cells for subchondral bone marrow lesions: From bench to bedside.Bone Rep. 2022 Oct 21;17:101630. doi: 10.1016/j.bonr.2022.101630. eCollection 2022 Dec. Bone Rep. 2022. PMID: 36310763 Free PMC article.
-
Navigating the Therapeutic Landscape: A Comprehensive Review of Platelet-Rich Plasma and Bone Marrow Aspirate Concentrate in Knee Osteoarthritis.Cureus. 2024 Feb 23;16(2):e54747. doi: 10.7759/cureus.54747. eCollection 2024 Feb. Cureus. 2024. PMID: 38524005 Free PMC article. Review.
-
Bone Marrow Aspirate Concentrate Injections for the Treatment of Knee Osteoarthritis: A Systematic Review of Randomized Controlled Trials.Orthop J Sports Med. 2024 Dec 4;12(12):23259671241296555. doi: 10.1177/23259671241296555. eCollection 2024 Dec. Orthop J Sports Med. 2024. PMID: 39640186 Free PMC article. Review.
-
Platelet Rich Plasma in the Repair of Articular Cartilage Injury: A Narrative Review.Cartilage. 2022 Jul-Sep;13(3):19476035221118419. doi: 10.1177/19476035221118419. Cartilage. 2022. PMID: 36086807 Free PMC article. Review.
References
-
- Kitaori T., Ito H., Schwarz E.M., Tsutsumi R., Yoshitomi H., Oishi S., Nakano M., Fujii N., Nagasawa T., Nakamura T. Stromal cell-derived factor 1/CXCR4 signaling is critical for the recruitment of mesenchymal stem cells to the fracture site during skeletal repair in a mouse model. Arthritis Rheum. 2009;60:813–823. doi: 10.1002/art.24330. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

