Electrochemistry/Photoelectrochemistry-Based Immunosensing and Aptasensing of Carcinoembryonic Antigen
- PMID: 34833818
- PMCID: PMC8624776
- DOI: 10.3390/s21227742
Electrochemistry/Photoelectrochemistry-Based Immunosensing and Aptasensing of Carcinoembryonic Antigen
Abstract
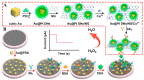
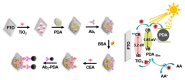
Recently, electrochemistry- and photoelectrochemistry-based biosensors have been regarded as powerful tools for trace monitoring of carcinoembryonic antigen (CEA) due to the fact of their intrinsic advantages (e.g., high sensitivity, excellent selectivity, small background, and low cost), which play an important role in early cancer screening and diagnosis and benefit people's increasing demands for medical and health services. Thus, this mini-review will introduce the current trends in electrochemical and photoelectrochemical biosensors for CEA assay and classify them into two main categories according to the interactions between target and biorecognition elements: immunosensors and aptasensors. Some recent illustrative examples are summarized for interested readers, accompanied by simple descriptions of the related signaling strategies, advanced materials, and detection modes. Finally, the development prospects and challenges of future electrochemical and photoelectrochemical biosensors are considered.
Keywords: CEA; aptasensor; electrochemistry; immunosensor; photoelectrochemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures










References
-
- Sadighbayan D., Sadighbayan K., Khosroushahi A.Y., Hasanzadeh M. Recent advances on the DNA-based electrochemical biosensing of cancer biomarkers: Analytical approach. Trends Anal. Chem. 2019;119:115609. doi: 10.1016/j.trac.2019.07.020. - DOI
-
- Zhao Y., Tan L., Jie G.F. Ultrasensitive electrochemiluminescence biosensor for the detection of carcinoembryonic antigen based on multiple amplification and a DNA walker. Sens. Actuators B Chem. 2021;333:129586. doi: 10.1016/j.snb.2021.129586. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

