Low-Dimensional Architectures in Isomeric cis-PtCl2{Ph2PCH2N(Ar)CH2PPh2} Complexes Using Regioselective-N(Aryl)-Group Manipulation
- PMID: 34833902
- PMCID: PMC8618721
- DOI: 10.3390/molecules26226809
Low-Dimensional Architectures in Isomeric cis-PtCl2{Ph2PCH2N(Ar)CH2PPh2} Complexes Using Regioselective-N(Aryl)-Group Manipulation
Abstract
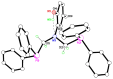
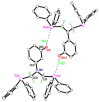
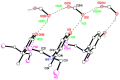
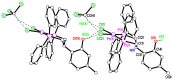
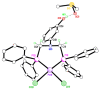
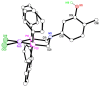
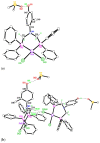
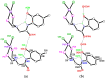
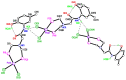
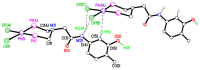
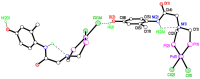
The solid-state behaviour of two series of isomeric, phenol-substituted, aminomethylphosphines, as the free ligands and bound to PtII, have been extensively studied using single crystal X-ray crystallography. In the first library, isomeric diphosphines of the type Ph2PCH2N(Ar)CH2PPh2 [1a-e; Ar = C6H3(Me)(OH)] and, in the second library, amide-functionalised, isomeric ligands Ph2PCH2N{CH2C(O)NH(Ar)}CH2PPh2 [2a-e; Ar = C6H3(Me)(OH)], were synthesised by reaction of Ph2PCH2OH and the appropriate amine in CH3OH, and isolated as colourless solids or oils in good yield. The non-methyl, substituted diphosphines Ph2PCH2N{CH2C(O)NH(Ar)}CH2PPh2 [2f, Ar = 3-C6H4(OH); 2g, Ar = 4-C6H4(OH)] and Ph2PCH2N(Ar)CH2PPh2 [3, Ar = 3-C6H4(OH)] were also prepared for comparative purposes. Reactions of 1a-e, 2a-g, or 3 with PtCl2(η4-cod) afforded the corresponding square-planar complexes 4a-e, 5a-g, and 6 in good to high isolated yields. All new compounds were characterised using a range of spectroscopic (1H, 31P{1H}, FT-IR) and analytical techniques. Single crystal X-ray structures have been determined for 1a, 1b∙CH3OH, 2f∙CH3OH, 2g, 3, 4b∙(CH3)2SO, 4c∙CHCl3, 4d∙½Et2O, 4e∙½CHCl3∙½CH3OH, 5a∙½Et2O, 5b, 5c∙¼H2O, 5d∙Et2O, and 6∙(CH3)2SO. The free phenolic group in 1b∙CH3OH, 2f∙CH3OH,2g, 4b∙(CH3)2SO, 5a∙½Et2O, 5c∙¼H2O, and 6∙(CH3)2SO exhibits various intra- or intermolecular O-H∙∙∙X (X = O, N, P, Cl) hydrogen contacts leading to different packing arrangements.
Keywords: P-ligands; amide groups; isomers; late-transition metals; phenols; secondary interactions; single crystal X-ray crystallography.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Lehn J.-M. Supramolecular Chemistry-Scope and Perspectives. Molecules, Supermolecules, and Molecular Devices (Nobel Lecture) Angew. Chem. Int. Ed. Engl. 1988;27:89–112. doi: 10.1002/anie.198800891. - DOI
-
- Daubignard J., Detz R.J., de Bruin B., Reek J.N.H. Phosphine Oxide Based Supramolecular Ligands in the Rhodium-Catalysed Asymmetric Hydrogenation. Organometallics. 2019;38:3961–3969. doi: 10.1021/acs.organomet.9b00484. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

