Synthesis of Purine-Based Ionic Liquids and Their Applications
- PMID: 34834050
- PMCID: PMC8620494
- DOI: 10.3390/molecules26226958
Synthesis of Purine-Based Ionic Liquids and Their Applications
Abstract
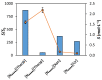
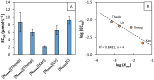
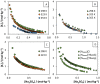
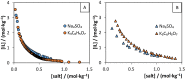
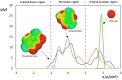
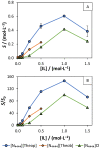
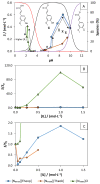
Bio-based ionic liquids (ILs) are being increasingly sought after, as they are more sustainable and eco-friendly. Purines are the most widely distributed, naturally occurring N-heterocycles, but their low water-solubility limits their application. In this work, four purines (theobromine, theophylline, xanthine, and uric acid) were combined with the cation tetrabutylammonium to synthesize bio-based ILs. The physico-chemical properties of the purine-based ILs were characterized, including their melting and decomposition temperatures and water-solubility. The ecotoxicity against the microalgae Raphidocelis subcapitata was also determined. The ILs show good thermal stability (>457 K) and an aqueous solubility enhancement ranging from 53- to 870-fold, in comparison to their respective purine percursors, unlocking new prospects for their application where aqueous solutions are demanded. The ecotoxicity of these ILs seems to be dominated by the cation, and it is similar to chloride-based IL, emphasizing that the use of natural anions does not necessarily translate to more benign ILs. The application of the novel ILs in the formation of aqueous biphasic systems (ABS), and as solubility enhancers, was also evaluated. The ILs were able to form ABS with sodium sulfate and tripotassium citrate salts. The development of thermoresponsive ABS, using sodium sulfate as a salting-out agent, was accomplished, with the ILs having different thermosensitivities. In addition, the purine-based ILs acted as solubility enhancers of ferulic acid in aqueous solution.
Keywords: ecotoxicity; liquid–liquid equilibrium; solubility; synthesis; thermoresponsive systems.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Walden P. Molecular weights and electrical conductivity of several fused salts. Bull. Acad. Imper. Sci. 1914;8:405–422.
-
- Mariani A., Bonomo M., Gao X., Centrella B., Nucara A., Buscaino R., Barge A., Barbero N., Gontrani L., Passerini S. The unseen evidence of Reduced Ionicity: The elephant in (the) room temperature ionic liquids. J. Mol. Liq. 2021;324:115069. doi: 10.1016/j.molliq.2020.115069. - DOI
-
- Singh S.K., Savoy A.W. Ionic liquids synthesis and applications: An overview. J. Mol. Liq. 2020;297:112038. doi: 10.1016/j.molliq.2019.112038. - DOI
MeSH terms
Substances
Grants and funding
- UIDB/50011/2020, UIDP/50011/2020, UIDP/50017/2020, UIDB/50017/2020/Fundação para a Ciência e Tecnologia
- Portuguese-French program Hubert Curien (Pessoa)/Fundação para a Ciência e Tecnologia
- SFRH/BD/143612/2019/Fundação para a Ciência e Tecnologia
- SFRH/BD/147346/2019/Fundação para a Ciência e Tecnologia
- CEECIND/00831/2017/Fundação para a Ciência e Tecnologia
LinkOut - more resources
Full Text Sources

