Phytochemistry, Pharmacology, and Nutraceutical Profile of Carissa Species: An Updated Review
- PMID: 34834102
- PMCID: PMC8624575
- DOI: 10.3390/molecules26227010
Phytochemistry, Pharmacology, and Nutraceutical Profile of Carissa Species: An Updated Review
Abstract
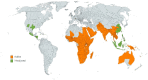
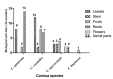
Carissa, a genus of the Apocynaceae family, consists of evergreen species, such as shrubs as well as small trees that are native to Asia, Africa, and Oceania's subtropical and tropical regions. Most of the Carissa species are traditionally used to treat various diseases, such as chest pain, headaches, gonorrhoea, rheumatism, syphilis, oedema, rabies, stomach pain, hepatitis, cardiac diseases, and asthma. The pharmacological studies on Carissa species revealed its antioxidant, antimicrobial, anticancer, cardioprotective, antipyretic, analgesic, wound healing, anticonvulsant, antiarthritic, adaptogenic, anti-inflammatory, and antidiabetic activities, thus validating its use in indigenous medicine systems. The review article summarised the comprehensive literature available, including morphology, indigenous uses, bioactive composition, nutraceutical, and pharmacological activities of Carissa species. A total of 155 research papers were cited in this review article. The Carissa fruits are rich in dietary fibre, lipids, proteins, carbohydrates, vitamin C, and macro- and micro-elements. A total of 121 compounds (35 polyphenols (flavonoids and phenolic acids), 30 lignans, 41 terpenoids, 7 steroids, 2 coumarins, and 6 cardiac glycosides) have been extracted from C. spinarum, C. carandas, and C. macrocarpa. Among all chemical constituents, lupeol, carissol, naringin, carisssone, scopoletin, carissaeduloside A, D, J, carandinol, sarhamnoloside, carissanol, olivil, carinol, 3β-hydroxyolean-11-en-28,13β-oilde, ursolic acid, and carissone are the key bioactive constituents responsible for pharmacological activities of genus Carissa. The gathered ethnopharmacological information in the review will help to understand the therapeutic relevance of Carissa as well as paving a way for further exploration in the discovery of novel plant-based drugs.
Keywords: Carissa; bioactive compounds; nutraceutical profile; pharmacological activity; phytochemistry.
Conflict of interest statement
The authors declare no conflict of interest in the publication.
Figures





References
-
- The Plant List. Version 1. [(accessed on 1 January 2010)]. Available online: http://www.theplantlist.org.
-
- Ngulde S.I., Sandabe U.K., Tijjani M.B., Barkindo A.A., Hussaini I.M. Phytochemical constituents antimicrobial screening and acute toxicity studies of the ethanol extract of Carissa edulis Vahl root bark in rats and mice. Am. J. Res. Commun. 2013;1:99–110.
-
- Fanta Yadang S.A., Taiwe Sotoing G., Ngatcha Zouakeu K.S., Khan M.A., Agbor G.A., Ur-Rahman N., Ngo Bum E. Quantification of bioactive compounds and evaluation of the antioxidant activity of Carissa edulis Vahl. (Apocynaceae) leaves. Sci. World J. 2019;2019:7549620. doi: 10.1155/2019/7549620. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

