Carbonic Anhydrase Inhibition with Sulfonamides Incorporating Pyrazole- and Pyridazinecarboxamide Moieties Provides Examples of Isoform-Selective Inhibitors
- PMID: 34834114
- PMCID: PMC8625619
- DOI: 10.3390/molecules26227023
Carbonic Anhydrase Inhibition with Sulfonamides Incorporating Pyrazole- and Pyridazinecarboxamide Moieties Provides Examples of Isoform-Selective Inhibitors
Abstract
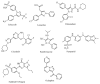
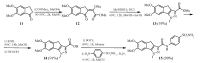
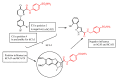
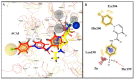
A series of benzenesulfonamides incorporating pyrazole- and pyridazinecarboxamides decorated with several bulky moieties has been obtained by original procedures. The new derivatives were investigated for the inhibition of four physiologically crucial human carbonic anhydrase (hCA, EC 4.2.2.1.1) isoforms, hCA I and II (cytosolic enzymes) as well as hCA IX and XII (transmembrane, tumor-associated isoforms). Examples of isoform-selective inhibitors were obtained for all four enzymes investigated here, and a computational approach was employed for explaining the observed selectivity, which may be useful in drug design approaches for obtaining inhibitors with pharmacological applications useful as antiglaucoma, diuretic, antitumor or anti-cerebral ischemia drugs.
Keywords: carbonic anhydrase; inhibitors; metalloenzymes; pyrazole derivatives.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

