Activators and Inhibitors of Protein Kinase C (PKC): Their Applications in Clinical Trials
- PMID: 34834162
- PMCID: PMC8621927
- DOI: 10.3390/pharmaceutics13111748
Activators and Inhibitors of Protein Kinase C (PKC): Their Applications in Clinical Trials
Abstract
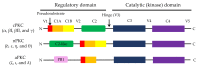
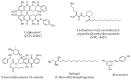
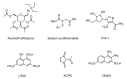
Protein kinase C (PKC), a family of phospholipid-dependent serine/threonine kinase, is classed into three subfamilies based on their structural and activation characteristics: conventional or classic PKC isozymes (cPKCs; α, βI, βII, and γ), novel or non-classic PKC isozymes (nPKCs; δ, ε, η, and θ), and atypical PKC isozymes (aPKCs; ζ, ι, and λ). PKC inhibitors and activators are used to understand PKC-mediated intracellular signaling pathways and for the diagnosis and treatment of various PKC-associated diseases, such as cancers, neurological diseases, cardiovascular diseases, and infections. Many clinical trials of PKC inhibitors in cancers showed no significant clinical benefits, meaning that there is a limitation to design a cancer therapeutic strategy targeting PKC alone. This review will focus on the activators and inhibitors of PKC and their applications in clinical trials.
Keywords: activator; cancer; clinical trial; inhibitor; protein kinase C; signaling pathway.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Protein Kinase C (PKC) Isozymes as Diagnostic and Prognostic Biomarkers and Therapeutic Targets for Cancer.Cancers (Basel). 2022 Nov 3;14(21):5425. doi: 10.3390/cancers14215425. Cancers (Basel). 2022. PMID: 36358843 Free PMC article. Review.
-
Compartmentalized protein kinase C activation in ovarian carcinoma cells.Methods Mol Med. 2001;39:621-31. doi: 10.1385/1-59259-071-3:621. Methods Mol Med. 2001. PMID: 21340822
-
Ischemic preconditioning induces selective translocation of protein kinase C isoforms epsilon and eta in the heart of conscious rabbits without subcellular redistribution of total protein kinase C activity.Circ Res. 1997 Sep;81(3):404-14. doi: 10.1161/01.res.81.3.404. Circ Res. 1997. PMID: 9285643
-
Protein kinase C delta.Eur J Biochem. 1999 Feb;259(3):555-64. doi: 10.1046/j.1432-1327.1999.00120.x. Eur J Biochem. 1999. PMID: 10092837 Review.
-
Determination of the specific substrate sequence motifs of protein kinase C isozymes.J Biol Chem. 1997 Jan 10;272(2):952-60. doi: 10.1074/jbc.272.2.952. J Biol Chem. 1997. PMID: 8995387
Cited by
-
NCX2 Regulates Intracellular Calcium Homeostasis and Translocation of HIF-1α into the Nucleus to Inhibit Glioma Invasion.Biochem Genet. 2023 Jun;61(3):979-994. doi: 10.1007/s10528-022-10274-9. Epub 2022 Nov 5. Biochem Genet. 2023. PMID: 36334237 Free PMC article.
-
In silico and in vitro study of FLT3 inhibitors and their application in acute myeloid leukemia.Mol Med Rep. 2024 Dec;30(6):229. doi: 10.3892/mmr.2024.13353. Epub 2024 Oct 11. Mol Med Rep. 2024. PMID: 39392050 Free PMC article.
-
Screening of crosstalk and pyroptosis-related genes linking periodontitis and osteoporosis based on bioinformatics and machine learning.Front Immunol. 2022 Aug 5;13:955441. doi: 10.3389/fimmu.2022.955441. eCollection 2022. Front Immunol. 2022. PMID: 35990678 Free PMC article.
-
Protein Kinase C at the Crossroad of Mutations, Cancer, Targeted Therapy and Immune Response.Biology (Basel). 2023 Jul 26;12(8):1047. doi: 10.3390/biology12081047. Biology (Basel). 2023. PMID: 37626933 Free PMC article. Review.
-
Diabetic peripheral neuropathy: pathogenetic mechanisms and treatment.Front Endocrinol (Lausanne). 2024 Jan 9;14:1265372. doi: 10.3389/fendo.2023.1265372. eCollection 2023. Front Endocrinol (Lausanne). 2024. PMID: 38264279 Free PMC article. Review.
References
-
- Kang J.H. Protein kinase c (PKC) isozymes and cancer. New J. Sci. 2014;2014:231418. doi: 10.1155/2014/231418. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

