Green Synthesized Silver Nanoparticles Using Tridax Procumbens for Topical Application: Excision Wound Model and Histopathological Studies
- PMID: 34834169
- PMCID: PMC8623640
- DOI: 10.3390/pharmaceutics13111754
Green Synthesized Silver Nanoparticles Using Tridax Procumbens for Topical Application: Excision Wound Model and Histopathological Studies
Abstract
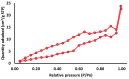
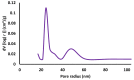
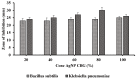
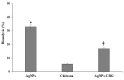
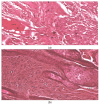
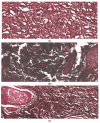
The objective of this study was to synthesize silver nanoparticles from the leaves of Tridax procumbens and develop its topical gels using chitosan to investigate the wound healing efficacy concomitant with the histopathological study. Green synthesized silver nanoparticles (AgNPs) were prepared by reacting silver nitrate (0.3 M) with leaf extract and characterized by particle analysis, FTIR, XRD, SEM, BET, and TGA. The results revealed formed AgNPs were nano-sized (138 ± 2.1 nm), monodispersed (PDI: 0.460 ± 0.3), inter-particle repulsion (zeta: -20.4 ± 5.20 mV), stabilized, crystalline and, spherical with size ranging from 80-100 nm as per SEM micro photos. The BET analysis of AgNPs presents the surface area (12.861 m2/g), pore volume (0.037 cc/g), and pore radius (24.50 nm).TGA results show a loss of 13.39% up to 300 °C. The topical formulation was developed by loading AgNPs in chitosan-based gels, evaluated by pH, thermal cycling, centrifugal, and spreadability tests. AgNPs chitosan gels results showed skin compatibility, higher stability, and spreading ability. The maximum antibacterial zone of inhibition was found to be 25 ± 0.98 mm for bacillus subtitles and 30 ± 1.99 mm for Klebsiella pneumoniae, respectively. Nanosilver-containing gel also showed excellent compatibility with erythrocytes. Excision wound model was used to assess the wound healing property of the developed AgNP gels, the results of which indicated a significantly progressive healing process in test-group of animals treated with chitosan-based gels containing AgNPs. A histopathological study further confirmed the almost normal skin structure of treated animal tissue compared to standard and negative control. Thus, green synthesized AgNPs loaded chitosan-based topical gel can potentially be used for wound healing application.
Keywords: green synthesis; histopathology; leaf extract; silver nanoparticles; tridax procumbens; wound healing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures











References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

