Artepillin C Reduces Allergic Airway Inflammation by Induction of Monocytic Myeloid-Derived Suppressor Cells
- PMID: 34834178
- PMCID: PMC8625726
- DOI: 10.3390/pharmaceutics13111763
Artepillin C Reduces Allergic Airway Inflammation by Induction of Monocytic Myeloid-Derived Suppressor Cells
Abstract
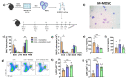
Propolis is a natural product produced by bees that is primarily used in complementary and alternative medicine and has anti-inflammatory, antibacterial, antiviral, and antitumoral biological properties. Some studies have reported the beneficial effects of propolis in models of allergic asthma. In a previous study, our group showed that green propolis treatment reduced airway inflammation and mucus secretion in an ovalbumin (OVA)-induced asthma model and resulted in increased regulatory T cells (Treg) and polymorphonuclear myeloid-derived suppressor cells (PMN-MDSC) frequencies in the lungs, two leukocyte populations that have immunosuppressive functions. In this study, we evaluated the anti-inflammatory effects of artepillin C (ArtC), the major compound of green propolis, in the context of allergic airway inflammation. Our results show that ArtC induces in vitro differentiation of Treg cells and monocytic MDSC (M-MDSC). Furthermore, in an OVA-induced asthma model, ArtC treatment reduced pulmonary inflammation, eosinophil influx to the airways, mucus and IL-5 secretion along with increased frequency of M-MDSC, but not Treg cells, in the lungs. Using an adoptive transfer model, we confirmed that the effect of ArtC in the reduction in airway inflammation was dependent on M-MDSC. Altogether, our data show that ArtC exhibits an anti-inflammatory effect and might be an adjuvant therapy for allergic asthma.
Keywords: M-MDSC; allergic asthma; artepillin C; propolis; therapies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Machado J.L., Assunção A.K.M., Da Silva M.C.P., Dos Reis A.S., Costa G.C., Arruda D.D.S., Rocha B.A., Vaz M.M., Paes A.M., Guerra R.N.M., et al. Brazilian green propolis: Anti-inflammatory property by an immunomodulatory activity. Evid.-Based Complement. Altern. Med. 2012;2012:157652. doi: 10.1155/2012/157652. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

