Circulating Tumour Cells (CTCs) in NSCLC: From Prognosis to Therapy Design
- PMID: 34834295
- PMCID: PMC8619417
- DOI: 10.3390/pharmaceutics13111879
Circulating Tumour Cells (CTCs) in NSCLC: From Prognosis to Therapy Design
Abstract
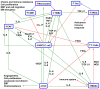
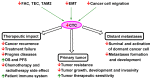
Designing optimal (neo)adjuvant therapy is a crucial aspect of the treatment of non-small-cell lung carcinoma (NSCLC). Standard methods of chemotherapy, radiotherapy, and immunotherapy represent effective strategies for treatment. However, in some cases with high metastatic activity and high levels of circulating tumour cells (CTCs), the efficacy of standard treatment methods is insufficient and results in treatment failure and reduced patient survival. CTCs are seen not only as an isolated phenomenon but also a key inherent part of the formation of metastasis and a key factor in cancer death. This review discusses the impact of NSCLC therapy strategies based on a meta-analysis of clinical studies. In addition, possible therapeutic strategies for repression when standard methods fail, such as the administration of low-toxicity natural anticancer agents targeting these phenomena (curcumin and flavonoids), are also discussed. These strategies are presented in the context of key mechanisms of tumour biology with a strong influence on CTC spread and metastasis (mechanisms related to tumour-associated and -infiltrating cells, epithelial-mesenchymal transition, and migration of cancer cells).
Keywords: CTCs; NSCLCs; curcumin; flavonoids; metastasis suppression.
Conflict of interest statement
All authors declare no conflict of interest. The funders had no role in the review topic choice, writing of the manuscript, or decision to publish the results.
Figures



Similar articles
-
Mesenchymal circulating tumor cells (CTCs) and OCT4 mRNA expression in CTCs for prognosis prediction in patients with non-small-cell lung cancer.Clin Transl Oncol. 2017 Sep;19(9):1147-1153. doi: 10.1007/s12094-017-1652-z. Epub 2017 Apr 3. Clin Transl Oncol. 2017. PMID: 28374320
-
Detection of circulating tumour cells with a hybrid (epithelial/mesenchymal) phenotype in patients with metastatic non-small cell lung cancer.Br J Cancer. 2011 Oct 25;105(9):1338-41. doi: 10.1038/bjc.2011.405. Epub 2011 Oct 4. Br J Cancer. 2011. PMID: 21970878 Free PMC article.
-
The prognostic and therapeutic implications of circulating tumor cell phenotype detection based on epithelial-mesenchymal transition markers in the first-line chemotherapy of HER2-negative metastatic breast cancer.Cancer Commun (Lond). 2019 Jan 3;39(1):1. doi: 10.1186/s40880-018-0346-4. Cancer Commun (Lond). 2019. PMID: 30606259 Free PMC article. Clinical Trial.
-
Circulating tumour cells in metastatic head and neck cancers.Int J Cancer. 2015 Jun 1;136(11):2515-23. doi: 10.1002/ijc.29108. Epub 2014 Aug 11. Int J Cancer. 2015. PMID: 25111594 Review.
-
Circulating tumour cells, their role in metastasis and their clinical utility in lung cancer.Lung Cancer. 2012 Apr;76(1):19-25. doi: 10.1016/j.lungcan.2011.10.018. Epub 2011 Dec 29. Lung Cancer. 2012. PMID: 22209049 Review.
Cited by
-
Cancer Stem Cells (CSCs), Circulating Tumor Cells (CTCs) and Their Interplay with Cancer Associated Fibroblasts (CAFs): A New World of Targets and Treatments.Cancers (Basel). 2022 May 13;14(10):2408. doi: 10.3390/cancers14102408. Cancers (Basel). 2022. PMID: 35626011 Free PMC article. Review.
-
Novel Anticancer Strategies II.Pharmaceutics. 2023 Feb 10;15(2):605. doi: 10.3390/pharmaceutics15020605. Pharmaceutics. 2023. PMID: 36839927 Free PMC article.
-
The Role of IL-6 in Cancer Cell Invasiveness and Metastasis-Overview and Therapeutic Opportunities.Cells. 2022 Nov 21;11(22):3698. doi: 10.3390/cells11223698. Cells. 2022. PMID: 36429126 Free PMC article. Review.
-
Investigating antibacterial and anti-inflammatory properties of synthetic curcuminoids.Front Med (Lausanne). 2024 Oct 29;11:1478122. doi: 10.3389/fmed.2024.1478122. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39534226 Free PMC article.
-
Curcumin: A Potential Weapon in the Prevention and Treatment of Head and Neck Cancer.ACS Pharmacol Transl Sci. 2024 Oct 14;7(11):3394-3418. doi: 10.1021/acsptsci.4c00518. eCollection 2024 Nov 8. ACS Pharmacol Transl Sci. 2024. PMID: 39539276 Review.
References
-
- Postmus P.E., Kerr K.M., Oudkerk M., Senan S., Waller D.A., Vansteenkiste J., Escriu C., Peters S. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017;28:iv1–iv21. doi: 10.1093/annonc/mdx222. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

