Understanding the Importance of Capsules in Dry Powder Inhalers
- PMID: 34834351
- PMCID: PMC8623721
- DOI: 10.3390/pharmaceutics13111936
Understanding the Importance of Capsules in Dry Powder Inhalers
Abstract
Pulmonary drug delivery is currently the focus of research and development because of its potential to produce maximum therapeutic benefit to patients by directing the drug straight to the lung disease site. Among all the available delivery options, one popular, proven and convenient inhaler device is the capsule-based dry powder inhaler (cDPI) for the treatment of an increasingly diverse range of diseases. cDPIs use a hard capsule that contains a powder formulation which consists of a mixture of a micronized drug and a carrier usually the lactose, known for its good lung tolerance. The capsule is either inserted into the device during manufacturer or by the patient prior to use. After perforating, opening or cut the capsule in the device, patients take a deep and rapid breath to inhale the powder, using air as the vector of drug displacement. The system is simple, relatively cheap and characterized by a lower carbon footprint than that of pressurized metered dose inhalers. This article reviews cDPI technology, focusing particularly on the importance of capsule characteristics and their function as a drug reservoir in cDPIs.
Keywords: capsule; dry powder inhaler; inhalation; pulmonary drug delivery.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


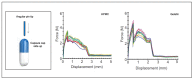
References
-
- Chierici V., Cavalieri L., Piraino A., Paleari D., Quarta E., Sonvico F., Melani A.S., Buttini F. Consequences of Not-Shaking and Shake-Fire Delays on the Emitted Dose of Some Commercial Solution and Suspension Pressurized Metered Dose Inhalers. Expert Opin. Drug Del. 2020;17:1025–1039. doi: 10.1080/17425247.2020.1767066. - DOI - PubMed
-
- Cocozza S. Inhaling Device for Medial Powder Compositions. 3807400. U.S. Patent. 1974 April 30;
Publication types
LinkOut - more resources
Full Text Sources

