Modulating the Blood-Brain Barrier: A Comprehensive Review
- PMID: 34834395
- PMCID: PMC8618722
- DOI: 10.3390/pharmaceutics13111980
Modulating the Blood-Brain Barrier: A Comprehensive Review
Abstract
The highly secure blood-brain barrier (BBB) restricts drug access to the brain, limiting the molecular toolkit for treating central nervous system (CNS) diseases to small, lipophilic drugs. Development of a safe and effective BBB modulator would revolutionise the treatment of CNS diseases and future drug development in the area. Naturally, the field has garnered a great deal of attention, leading to a vast and diverse range of BBB modulators. In this review, we summarise and compare the various classes of BBB modulators developed over the last five decades-their recent advancements, advantages and disadvantages, while providing some insight into their future as BBB modulators.
Keywords: BBB modulation; BBB permeability; CNS drug delivery; alzheimers; blood-brain barrier (BBB); focused ultrasound; glioblastoma; hyperosmolar agents; intra-arterial drug delivery; tight junction (TJ).
Conflict of interest statement
The authors declare no conflict of interest.
Figures




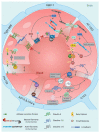
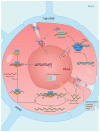
References
-
- Atkinson F., Cole S., Green C., van de Waterbeemd H. Lipophilicity and Other Parameters Affecting Brain Penetration. Curr. Med. Chem. Nerv. Syst. Agents. 2002;2:229–240. doi: 10.2174/1568015023358058. - DOI
-
- Hansch C., Leo A. Substituent Constants for Correlation Analysis in Chemistry and Biology. Wiley; Hoboken, NJ, USA: 1979. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

