Morphology and Anatomy of Branch-Branch Junctions in Opuntia ficus-indica and Cylindropuntia bigelovii: A Comparative Study Supported by Mechanical Tissue Quantification
- PMID: 34834679
- PMCID: PMC8618873
- DOI: 10.3390/plants10112313
Morphology and Anatomy of Branch-Branch Junctions in Opuntia ficus-indica and Cylindropuntia bigelovii: A Comparative Study Supported by Mechanical Tissue Quantification
Abstract
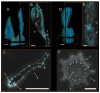
The Opuntioideae include iconic cacti whose lateral branch-branch junctions are intriguing objects from a mechanical viewpoint. We have compared Opuntia ficus-indica, which has stable branch connections, with Cylindropuntia bigelovii, whose side branches abscise under slight mechanical stress. To determine the underlying structures and mechanical characteristics of these stable versus shedding cacti junctions, we conducted magnetic resonance imaging, morphometric and anatomical analyses of the branches and tensile tests of individual tissues. The comparison revealed differences in geometry, shape and material properties as follows: (i) a more pronounced tapering of the cross-sectional area towards the junctions supports the abscission of young branches of C. bigelovii. (ii) Older branches of O. ficus-indica form, initially around the branch-branch junctions, collar-shaped periderm tissue. This secondary coverage mechanically stiffens the dermal tissue, giving a threefold increase in strength and a tenfold increase in the elastic modulus compared with the epidermis. (iii) An approximately 200-fold higher elastic modulus of the vascular bundles of O. ficus-indica is a prerequisite for the stable junction of its young branches. Our results provide, for both biological and engineered materials systems, important insights into the geometric characteristics and mechanical properties of branching joints that are either stable or easily detachable.
Keywords: Opuntioideae; abscission; cacti; magnetic resonance imaging; periderm formation; tissue tensile testing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Nobel P.S. Cacti: Biology and Uses. University of California Press; Berkeley, CA, USA: 2002.
-
- Benson L. The Cacti of the United States and Canada. Stanford University Press; Stanford, CA, USA: 1982.
-
- Griffith M.P., Porter J.M. Phylogeny of Opuntioideae (Cactaceae) Int. J. Plant Sci. 2009;170:107–116. doi: 10.1086/593048. - DOI
-
- Conde L.F. Anatomical Comparisons of Five Species of Opuntia (Cactaceae) Ann. Mo. Bot. Gard. 1975;62:425. doi: 10.2307/2395206. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

