Structural Studies on the Shapeshifting Murine Norovirus
- PMID: 34834968
- PMCID: PMC8621758
- DOI: 10.3390/v13112162
Structural Studies on the Shapeshifting Murine Norovirus
Abstract
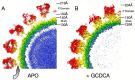
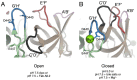
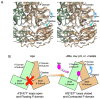
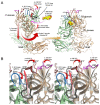
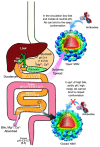
Noroviruses are responsible for almost a fifth of all cases of gastroenteritis worldwide. The calicivirus capsid is composed of 180 copies of VP1 with a molecular weight of ~58 kDa. This coat protein is divided into the N-terminus (N), the shell (S) and C-terminal protruding (P) domains. The S domain forms a shell around the viral RNA genome, while the P domains dimerize to form protrusions on the capsid surface. The P domain is subdivided into P1 and P2 subdomains, with the latter containing the binding sites for cellular receptors and neutralizing antibodies. Reviewed here are studies on murine norovirus (MNV) showing that the capsid responds to several physiologically relevant cues; bile, pH, Mg2+, and Ca2+. In the initial site of infection, the intestinal tract, high bile and metal concentrations and low pH cause two significant conformational changes: (1) the P domain contracts onto the shell domain and (2) several conformational changes within the P domain lead to enhanced receptor binding while blocking antibody neutralization. In contrast, the pH is neutral, and the concentrations of bile and metals are low in the serum. Under these conditions, the loops at the tip of the P domain are in the open conformation with the P domain floating on a linker or tether above the shell. This conformational state favors antibody binding but reduces interactions with the receptor. In this way, MNV uses metabolites and environmental cues in the intestine to optimize cellular attachment and escape antibody binding but presents a wholly different structure to the immune system in the serum. To our knowledge, this is the first example of a virus shapeshifting in this manner to escape the immune response.
Keywords: antibodies; bile; neutralization; norovirus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

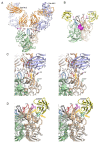
References
-
- Moe C.L., Sobsey M.D., Stewart P.W., Crawford-Brown D. Estimating the Risk of Human Calicivirus Infection from Drinking Water. International Workshop on HumanCaliciviruses; Atlanta, GA, USA: 1999. pp. 4–6.
-
- Blanton L.H., Adams S.M., Beard R.S., Wei G., Bulens S.N., Widdowson M.A., Glass R.I., Monroe S.S. Molecular and epidemiologic trends of caliciviruses associated with outbreaks of acute gastroenteritis in the United States, 2000–2004. J. Infect. Dis. 2006;193:413–421. doi: 10.1086/499315. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

