Peritoneal Administration of a Subunit Vaccine Encapsulated in a Nanodelivery System Not Only Augments Systemic Responses against SARS-CoV-2 but Also Stimulates Responses in the Respiratory Tract
- PMID: 34835008
- PMCID: PMC8617950
- DOI: 10.3390/v13112202
Peritoneal Administration of a Subunit Vaccine Encapsulated in a Nanodelivery System Not Only Augments Systemic Responses against SARS-CoV-2 but Also Stimulates Responses in the Respiratory Tract
Abstract
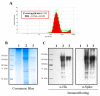
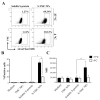
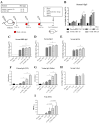
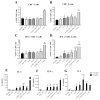
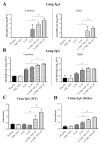
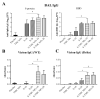
The COVID-19 pandemic has currently created an unprecedented threat to human society and global health. A rapid mass vaccination to create herd immunity against SARS-CoV-2 is a crucial measure to ease the spread of this disease. Here, we investigated the immunogenicity of a SARS-CoV-2 subunit vaccine candidate, a SARS-CoV-2 spike glycoprotein encapsulated in N,N,N-trimethyl chitosan particles or S-TMC NPs. Upon intraperitoneal immunization, S-TMC NP-immunized mice elicited a stronger systemic antibody response, with neutralizing capacity against SARS-CoV-2, than mice receiving the soluble form of S-glycoprotein. S-TMC NPs were able to stimulate the circulating IgG and IgA as found in SARS-CoV-2-infected patients. In addition, spike-specific T cell responses were drastically activated in S-TMC NP-immunized mice. Surprisingly, administration of S-TMC NPs via the intraperitoneal route also stimulated SARS-CoV-2-specific immune responses in the respiratory tract, which were demonstrated by the presence of high levels of SARS-CoV-2-specific IgG and IgA in the lung homogenates and bronchoalveolar lavages of the immunized mice. We found that peritoneal immunization with spike nanospheres stimulates both systemic and respiratory mucosal immunity.
Keywords: COVID-19 vaccine; SARS-CoV-2 spike glycoprotein; adjuvant delivery particles; mucosal immunity; peritoneal immunization.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Tabibzadeh A., Esghaei M., Soltani S., Yousefi P., Taherizadeh M., Tameshkel F.S., Golahdooz M., Panahi M., Ajdarkosh H., Zamani F., et al. Evolutionary study of COVID-19, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) as an emerging coronavirus: Phylogenetic analysis and literature review. Vet. Med. Sci. 2021;7:559–571. doi: 10.1002/vms3.394. - DOI - PMC - PubMed
-
- de Assis R.R., Jain A., Nakajima R., Jasinskas A., Felgner J., Obiero J.M., Norris P.J., Stone M., Simmons G., Bagri A., et al. Analysis of SARS-CoV-2 antibodies in COVID-19 convalescent blood using a coronavirus antigen microarray. Nat. Commun. 2021;12:6. doi: 10.1038/s41467-020-20095-2. - DOI - PMC - PubMed
-
- Piccoli L., Park Y.J., Tortorici M.A., Czudnochowski N., Walls A.C., Beltramello M., Silacci-Fregni C., Pinto D., Rosen L.E., Bowen J.E., et al. Mapping Neutralizing and Immunodominant Sites on the SARS-CoV-2 Spike Receptor-Binding Domain by Structure-Guided High-Resolution Serology. Cell. 2020;183:1024–1042.e21. doi: 10.1016/j.cell.2020.09.037. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

