Human Papillomaviruses-Associated Cancers: An Update of Current Knowledge
- PMID: 34835040
- PMCID: PMC8623401
- DOI: 10.3390/v13112234
Human Papillomaviruses-Associated Cancers: An Update of Current Knowledge
Abstract
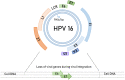
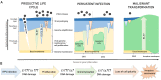
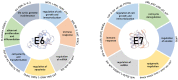
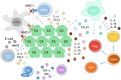
Human papillomaviruses (HPVs), which are small, double-stranded, circular DNA viruses infecting human epithelial cells, are associated with various benign and malignant lesions of mucosa and skin. Intensive research on the oncogenic potential of HPVs started in the 1970s and spread across Europe, including Croatia, and worldwide. Nowadays, the causative role of a subset of oncogenic or high-risk (HR) HPV types, led by HPV-16 and HPV-18, of different anogenital and head and neck cancers is well accepted. Two major viral oncoproteins, E6 and E7, are directly involved in the development of HPV-related malignancies by targeting synergistically various cellular pathways involved in the regulation of cell cycle control, apoptosis, and cell polarity control networks as well as host immune response. This review is aimed at describing the key elements in HPV-related carcinogenesis and the advances in cancer prevention with reference to past and on-going research in Croatia.
Keywords: biomarkers; cancer; epigenetics; human papillomaviruses (HPV); immunology; oncoproteins; proteomics; therapeutics.
Conflict of interest statement
The authors have no conflict of interest to declare. The funders had no role in the writing of the manuscript, or in the decision to publish the review.
Figures

References
-
- zur Hausen H. Infections Causing Human Cancer. Wiley-VCH verlag GmbH &, Co. KGaA, Weinheim; Württemberg, Germany: 2006.
-
- Jablonska S., Dabrowski J., Jakubowicz K. Epidermodysplasia Verruciformis as a Model in Studies on the Role of Papovaviruses in Oncogenesis. Cancer Res. 1972;32:583–589. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

