Dynamics of Neutralizing Antibody Responses Following Natural SARS-CoV-2 Infection and Correlation with Commercial Serologic Tests. A Reappraisal and Indirect Comparison with Vaccinated Subjects
- PMID: 34835135
- PMCID: PMC8621742
- DOI: 10.3390/v13112329
Dynamics of Neutralizing Antibody Responses Following Natural SARS-CoV-2 Infection and Correlation with Commercial Serologic Tests. A Reappraisal and Indirect Comparison with Vaccinated Subjects
Abstract
Neutralising antibodies (NAbs) represent the real source of protection against SARS-CoV-2 infections by preventing the virus from entering target cells. The gold standard in the detection of these antibodies is the plaque reduction neutralization test (PRNT). As these experiments must be done in a very secure environment, other techniques based on pseudoviruses: pseudovirus neutralization test (pVNT) or surrogate virus neutralization test (sVNT) have been developed. Binding assays, on the other hand, measure total antibodies or IgG, IgM, and IgA directed against one epitope of the SARS-CoV-2, independently of their neutralizing capacity. The aim of this study is to compare the performance of six commercial binding assays to the pVNT and sVNT. In this study, we used blood samples from a cohort of 62 RT-PCR confirmed COVID-19 patients. Based on the results of the neutralizing assays, adapted cut-offs for the binding assays were calculated. The use of these adapted cut-offs does not permit to improve the accuracy of the serological assays and we did not find an adapted cut-off able to improve the capacity of these tests to detect NAbs. For a part of the population, a longitudinal follow-up with at least two samples for the same patient was performed. From day 14 to day 291, more than 75% of the samples were positive for NAbs (n = 87/110, 79.1%). Interestingly, 6 months post symptoms onset, the majority of the samples (N = 44/52, 84.6%) were still positive for NAbs. This is in sharp contrast with the results we obtained 6 months post-vaccination in our cohort of healthcare workers who have received the two-dose regimens of BNT162b2. In this cohort of vaccinated subjects, 43% (n = 25/58) of the participants no longer exhibit NAbs activity 180 days after the administration of the first dose of BNT162b2.
Keywords: COVID-19; SARS-CoV-2; neutralizing antibody.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
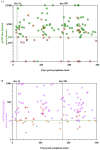
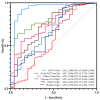
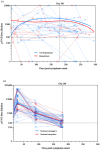
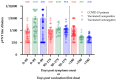
References
-
- Montesinos I., Dahma H., Wolff F., Dauby N., Delaunoy S., Wuyts M., Detemmerman C., Duterme C., Vandenberg O., Martin C., et al. Neutralizing antibody responses following natural SARS-CoV-2 infection: Dynamics and correlation with commercial serologic tests. J. Clin. Virol. 2021;144:104988. doi: 10.1016/j.jcv.2021.104988. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

