Alum Pickering Emulsion as Effective Adjuvant to Improve Malaria Vaccine Efficacy
- PMID: 34835175
- PMCID: PMC8624716
- DOI: 10.3390/vaccines9111244
Alum Pickering Emulsion as Effective Adjuvant to Improve Malaria Vaccine Efficacy
Abstract
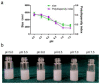
Malaria is a life-threatening global epidemic disease and has caused more than 400,000 deaths in 2019. To control and prevent malaria, the development of a vaccine is a potential method. An effective malaria vaccine should either combine antigens from all stages of the malaria parasite's life cycle, or epitopes of multiple key antigens due to the complexity of the Plasmodium parasite. Malaria's random constructed antigen-1 (M.RCAg-1) is one of the recombinant vaccines, which was selected from a DNA library containing thousands of diverse multi-epitope chimeric antigen genes. Moreover, besides selecting an antigen, using an adjuvant is another important procedure for most vaccine development procedures. Freund's adjuvant is considered an effective vaccine adjuvant for malaria vaccine, but it cannot be used in clinical settings because of its serious side effects. Traditional adjuvants, such as alum adjuvant, are limited by their unsatisfactory immune effects in malaria vaccines, hence there is an urgent need to develop a novel, safe and efficient adjuvant. In recent years, Pickering emulsions have attracted increasing attention as novel adjuvant. In contrast to classical emulsions, Pickering emulsions are stabilized by solid particles instead of surfactant, having pliability and lateral mobility. In this study, we selected aluminum hydroxide gel (termed as "alum") as a stabilizer to prepare alum-stabilized Pickering emulsions (ALPE) as a malaria vaccine adjuvant. In addition, monophosphoryl lipid A (MPLA) as an immunostimulant was incorporated into the Pickering emulsion (ALMPE) to further enhance the immune response. In vitro tests showed that, compared with alum, ALPE and ALMPE showed higher antigen load rates and could be effectively endocytosed by J774a.1 cells. In vivo studies indicated that ALMPE could induce as high antibody titers as Freund's adjuvant. The biocompatibility study also proved ALMPE with excellent biocompatibility. These results suggest that ALMPE is a potential adjuvant for a malaria vaccine.
Keywords: M.RCAg-1; adjuvant; alum stabilized Pickering emulsion; immune response; malaria vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Cationic Nanoparticle-Stabilized Vaccine Delivery System for the H9N2 Vaccine to Promote Immune Response in Chickens.Mol Pharm. 2023 Mar 6;20(3):1613-1623. doi: 10.1021/acs.molpharmaceut.2c00805. Epub 2023 Feb 16. Mol Pharm. 2023. PMID: 36795759
-
Poria cocos polysaccharide-loaded Alum Pickering emulsion as vaccine adjuvant to enhance immune responses.Colloids Surf B Biointerfaces. 2024 Dec;244:114144. doi: 10.1016/j.colsurfb.2024.114144. Epub 2024 Aug 6. Colloids Surf B Biointerfaces. 2024. PMID: 39116600
-
Augmenting vaccine efficacy: Tailored immune strategy with alum-stabilized Pickering emulsion.Vaccine. 2024 Sep 17;42(22):126022. doi: 10.1016/j.vaccine.2024.05.070. Epub 2024 Jun 13. Vaccine. 2024. PMID: 38876839
-
Use of novel adjuvants and delivery systems to improve the humoral and cellular immune response to malaria vaccine candidate antigens.Vaccine. 1993;11(5):591-3. doi: 10.1016/0264-410x(93)90239-t. Vaccine. 1993. PMID: 8488718 Review.
-
Adjuvants for vaccines to drugs of abuse and addiction.Vaccine. 2014 Sep 22;32(42):5382-9. doi: 10.1016/j.vaccine.2014.07.085. Epub 2014 Aug 8. Vaccine. 2014. PMID: 25111169 Free PMC article. Review.
Cited by
-
Development and Optimal Immune Strategy of an Alum-Stabilized Pickering emulsion for Cancer Vaccines.Vaccines (Basel). 2023 Jun 28;11(7):1169. doi: 10.3390/vaccines11071169. Vaccines (Basel). 2023. PMID: 37514985 Free PMC article.
-
Novel dual-pathogen multi-epitope mRNA vaccine development for Brucella melitensis and Mycobacterium tuberculosis in silico approach.PLoS One. 2024 Oct 28;19(10):e0309560. doi: 10.1371/journal.pone.0309560. eCollection 2024. PLoS One. 2024. PMID: 39466745 Free PMC article.
-
The Epidemiology of Influenza and the Associated Vaccines Development in China: A Review.Vaccines (Basel). 2022 Nov 6;10(11):1873. doi: 10.3390/vaccines10111873. Vaccines (Basel). 2022. PMID: 36366381 Free PMC article. Review.
-
Evaluation of immunoprotective effects of PlpE multi-epitope protein incorporated within the aluminum hydroxide-adjuvanted inactivated vaccine against Pasteurella multocida infection in chickens.Poult Sci. 2025 Jun 14;104(9):105426. doi: 10.1016/j.psj.2025.105426. Online ahead of print. Poult Sci. 2025. PMID: 40561824 Free PMC article.
-
Research progress on emulsion vaccine adjuvants.Heliyon. 2024 Jan 12;10(3):e24662. doi: 10.1016/j.heliyon.2024.e24662. eCollection 2024 Feb 15. Heliyon. 2024. PMID: 38317888 Free PMC article. Review.
References
-
- Guo F., Liu Y., Zhang C., Wang Q., Gao Y., Deng W., Wang H., Su Z. Stabilization of a chimeric malaria antigen in separation and purification through efficient inhibition of protease activity by imidazole. Process. Biochem. 2018;65:213–219. doi: 10.1016/j.procbio.2017.10.013. - DOI
LinkOut - more resources
Full Text Sources

