Predictors of the Therapeutic Response to Intralesional Bivalent HPV Vaccine in Wart Immunotherapy
- PMID: 34835211
- PMCID: PMC8621280
- DOI: 10.3390/vaccines9111280
Predictors of the Therapeutic Response to Intralesional Bivalent HPV Vaccine in Wart Immunotherapy
Abstract
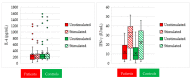
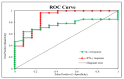
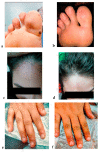
Variable intralesional immunotherapies have recently been proposed as a means of achieving a successful eradication of recurrent and recalcitrant human papillomavirus (HPV)-induced cutaneous and anogenital warts. The bivalent HPV vaccine is one of the newly proposed immunotherapeutic agents. We investigated the role of interleukin-4 (IL-4) and interferon-gamma (IFN-γ) as ex vivo immunologic predictors to estimate the response to the bivalent HPV vaccine as a potential immunotherapy for cutaneous and anogenital warts. Heparinized blood samples were withdrawn from forty patients with multiple recurrent recalcitrant cutaneous and anogenital warts and forty matched healthy control subjects. Whole blood cultures were prepared with and without bivalent HPV vaccine stimulation. Culture supernatants were harvested and stored for IL-4 and IFN-γ measurements using an enzyme-linked immunosorbent assay. A comparative analysis of IL-4 and IFN-γ levels in culture supernatants revealed a non-significant change between the patient and control groups. The bivalent HPV vaccine stimulated cultures exhibited a non-significant reduction in IL-4 levels within both groups. IFN-γ was markedly induced in both groups in response to bivalent HPV vaccine stimulation. The bivalent HPV vaccine can give a sensitive IFN-γ immune response ex vivo, superior to IL-4 and sufficient to predict both the successful eradication of HPV infection and the ultimate clearance of cutaneous and anogenital warts when the bivalent HPV vaccine immunotherapy is applied.
Keywords: anogenital warts; bivalent HPV vaccine; cutaneous warts; human papillomavirus; interferon-gamma; interleukin-4; wart immunotherapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Stanley M. Immune responses to human papilloma viruses. Indian J. Med. Res. 2009;130:266. - PubMed
-
- Renoux V.M., Bisig B., Langers I., Dortu E., Clémenceau B., Thiry M., Deroanne C., Colige A., Boniver J., Delvenne P. Human papillomavirus entry into NK cells requires CD16 expression and triggers cytotoxic activity and cytokine secretion. Eur. J. Immunol. 2011;41:3240–3252. doi: 10.1002/eji.201141693. - DOI - PubMed
LinkOut - more resources
Full Text Sources

