Vaccine versus Variants (3Vs): Are the COVID-19 Vaccines Effective against the Variants? A Systematic Review
- PMID: 34835238
- PMCID: PMC8622454
- DOI: 10.3390/vaccines9111305
Vaccine versus Variants (3Vs): Are the COVID-19 Vaccines Effective against the Variants? A Systematic Review
Abstract
Background: With the emergence and spread of new SARS-CoV-2 variants, concerns are raised about the effectiveness of the existing vaccines to protect against these new variants. Although many vaccines were found to be highly effective against the reference COVID-19 strain, the same level of protection may not be found against mutation strains. The objective of this study is to systematically review relevant studies in the literature and compare the efficacy of COVID-19 vaccines against new variants.
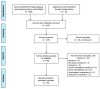
Methods: We conducted a systematic review of research published in Scopus, PubMed, and Google Scholar until 30 August 2021. Studies including clinical trials, prospective cohorts, retrospective cohorts, and test negative case-controls that reported vaccine effectiveness against any COVID-19 variants were considered. PRISMA recommendations were adopted for screening, eligibility, and inclusion.
Results: 129 unique studies were reviewed by the search criteria, of which 35 met the inclusion criteria. These comprised of 13 test negative case-control studies, 6 Phase 1-3 clinical trials, and 16 observational studies. The study location, type, vaccines used, variants considered, and reported efficacies were highlighted.
Conclusion: Full vaccination (two doses) offers strong protection against Alpha (B.1.1.7) with 13 out of 15 studies reporting more than 84% efficacy. The results are not conclusive against the Beta (B.1.351) variant for fully vaccinated individuals with 4 out of 7 studies reporting efficacies between 22 and 60% and 3 out of 7 studies reporting efficacies between 75 and 100%. Protection against Gamma (P.1) variant was lower than 50% according to two studies in fully vaccinated individuals. The data on Delta (B.1.617.2) variant is limited but indicates lower protection compared to other variants.
Keywords: COVID-19; SARS-CoV-2; vaccine effectiveness; vaccine efficacy; variants of concern.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- McKibbin W., Fernando R. Economics in the Time of COVID-19. CEPR Press; Washington, DC, USA: London, UK: 2020. The economic impact of COVID-19; p. 45.
-
- Chandir S., Siddiqi D.A., Mehmood M., Setayesh H., Siddique M., Mirza A., Soundardjee R., Dharma V.K., Shah M.T., Abdullah S., et al. Impact of COVID-19 pandemic response on uptake of routine immunizations in Sindh, Pakistan: An analysis of provincial electronic immunization registry data. Vaccine. 2020;38:7146–7155. doi: 10.1016/j.vaccine.2020.08.019. - DOI - PMC - PubMed
-
- Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., Angus B., Baillie V.L., Barnabas S.L., Bhorat Q.E., et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: A pooled analysis of four randomised trials. Lancet. 2021;397:881–891. doi: 10.1016/S0140-6736(21)00432-3. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous