Novel Cytoskeleton-Associated Proteins in Trypanosoma brucei Are Essential for Cell Morphogenesis and Cytokinesis
- PMID: 34835360
- PMCID: PMC8625193
- DOI: 10.3390/microorganisms9112234
Novel Cytoskeleton-Associated Proteins in Trypanosoma brucei Are Essential for Cell Morphogenesis and Cytokinesis
Abstract
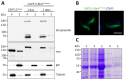
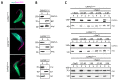
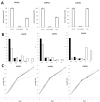
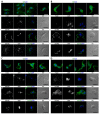
Trypanosome brucei, the causative agent of African sleeping sickness, harbours a highly ordered, subpellicular microtubule cytoskeleton that defines many aspects of morphology, motility and virulence. This array of microtubules is associated with a large number of proteins involved in its regulation. Employing proximity-dependent biotinylation assay (BioID) using the well characterised cytoskeleton-associated protein CAP5.5 as a probe, we identified CAP50 (Tb927.11.2610). This protein colocalises with the subpellicular cytoskeleton microtubules but not with the flagellum. Depletion by RNAi results in defects in cytokinesis, morphology and partial disorganisation of microtubule arrays. Published proteomics data indicate a possible association of CAP50 with two other, yet uncharacterised, cytoskeletal proteins, CAP52 (Tb927.6.5070) and CAP42 (Tb927.4.1300), which were therefore included in our analysis. We show that their depletion causes phenotypes similar to those described for CAP50 and that they are essential for cellular integrity.
Keywords: BioID; Trypanosoma brucei; cytoskeleton; mass spectrometry; microtubules.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources

