Development of Various Leishmania (Sauroleishmania) tarentolae Strains in Three Phlebotomus Species
- PMID: 34835382
- PMCID: PMC8622532
- DOI: 10.3390/microorganisms9112256
Development of Various Leishmania (Sauroleishmania) tarentolae Strains in Three Phlebotomus Species
Abstract
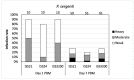
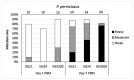
Leishmania (Sauroleishmania) tarentolae is transmitted by reptile-biting sand flies of the genus Sergentomyia, but the role of Phlebotomus sand flies in circulation of this parasite is unknown. Here, we compared the development of L. (S.) tarentolae strains in three Phlebotomus species: P. papatasi, P. sergenti, and P. perniciosus. Laboratory-bred sand flies were membrane-fed on blood with parasite suspension and dissected on days 1 and 7 post blood meal. Parasites were measured on Giemsa-stained gut smears and five morphological forms were distinguished. In all parasite-vector combinations, promastigotes were found in Malpighian tubules, often in high numbers, which suggests that this tissue is a typical location for L. (S.) tarentolae development in sand flies. All three studied strains colonized the hindgut, but also migrated anteriorly to both parts of the midgut and colonized the stomodeal valve. Significant differences were demonstrated between sand fly species: highest infection rates, high parasite loads, and the most frequent anterior migration with colonization of the stomodeal valve were found in P. perniciosus, while all these parameters were lowest in P. sergenti. In conclusion, the peripylarian type of development was demonstrated for three L. (S.) tarentolae strains in three Phlebotomus sand flies. We suggest paying more attention to Phlebotomus species, particularly P. perniciosus and P. papatasi, as potential secondary vectors of Sauroleishmania.
Keywords: Leishmania tarentolae; Phlebotomus; Sauroleishmania; experimental infections; sand flies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

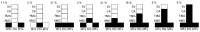
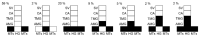


Similar articles
-
Experimental infections of sand flies and geckos with Leishmania (Sauroleishmania) adleri and Leishmania (S.) hoogstraali.Parasit Vectors. 2022 Aug 11;15(1):289. doi: 10.1186/s13071-022-05417-1. Parasit Vectors. 2022. PMID: 35953873 Free PMC article.
-
Leishmania (Sauroleishmania) tarentolae isolation and sympatric occurrence with Leishmania (Leishmania) infantum in geckoes, dogs and sand flies.PLoS Negl Trop Dis. 2022 Aug 9;16(8):e0010650. doi: 10.1371/journal.pntd.0010650. eCollection 2022 Aug. PLoS Negl Trop Dis. 2022. PMID: 35943980 Free PMC article.
-
Experimental feeding of Sergentomyia minuta on reptiles and mammals: comparison with Phlebotomus papatasi.Parasit Vectors. 2023 Apr 13;16(1):126. doi: 10.1186/s13071-023-05758-5. Parasit Vectors. 2023. PMID: 37055860 Free PMC article.
-
Midgut and stomodeal valve attachment of Leishmania in sand flies.Trends Parasitol. 2025 Jul 22:S1471-4922(25)00187-4. doi: 10.1016/j.pt.2025.06.017. Online ahead of print. Trends Parasitol. 2025. PMID: 40701917 Review.
-
Leishmania development in sand flies: parasite-vector interactions overview.Parasit Vectors. 2012 Dec 3;5:276. doi: 10.1186/1756-3305-5-276. Parasit Vectors. 2012. PMID: 23206339 Free PMC article. Review.
Cited by
-
Leishmania spp. in equids and their potential vectors in endemic areas of canine leishmaniasis.PLoS Negl Trop Dis. 2024 Jul 18;18(7):e0012290. doi: 10.1371/journal.pntd.0012290. eCollection 2024 Jul. PLoS Negl Trop Dis. 2024. PMID: 39024365 Free PMC article.
-
A novel chemically defined medium for the biotechnological and biomedical exploitation of the cell factory Leishmania tarentolae.Sci Rep. 2024 Apr 26;14(1):9562. doi: 10.1038/s41598-024-60383-1. Sci Rep. 2024. PMID: 38671070 Free PMC article.
-
Artificial blood-feeding of phlebotomines (Diptera: Psychodidae: Phlebotominae): is it time to repurpose biological membranes in light of ethical concerns?Parasit Vectors. 2022 Oct 31;15(1):399. doi: 10.1186/s13071-022-05511-4. Parasit Vectors. 2022. PMID: 36316748 Free PMC article.
-
Experimental infections of sand flies and geckos with Leishmania (Sauroleishmania) adleri and Leishmania (S.) hoogstraali.Parasit Vectors. 2022 Aug 11;15(1):289. doi: 10.1186/s13071-022-05417-1. Parasit Vectors. 2022. PMID: 35953873 Free PMC article.
-
Evolution of RNA viruses in trypanosomatids: new insights from the analysis of Sauroleishmania.Parasitol Res. 2023 Oct;122(10):2279-2286. doi: 10.1007/s00436-023-07928-x. Epub 2023 Jul 25. Parasitol Res. 2023. PMID: 37490143 Free PMC article.
References
-
- Ranque P. Ph.D. Thesis. Université d’Aix-Marseille II; Marseille, France: Apr 28, 1973. Étude morphologique et biologique de quelques Trypanosomidés récoltés au Sénégal. .
-
- Saf’janova V.M. The Leishmanias; Protozoology, Academy of Sciences. Volume 7. USSR All Union Society of Protozoologists; Leningrad, Russia: 1982. The problem of taxonomy with Leishmania; pp. 95–101.
-
- Killick-Kendrick R., Lainson R., Rioux J.A., Saf’janova V.M. The taxonomy of Leishmania-like parasites of reptiles. In: Rioux J.A., editor. Leishmania: Taxonomie et Phylogenèse. Applications Eco-epidéemiologiques (Colloque international du CNRS/INSERM, 1984), IMEE; Montpelier, France: 1986. pp. 143–148.
Grants and funding
LinkOut - more resources
Full Text Sources

